Method for electrocatalytically reducing carbon dioxide based on chip on flex (COF)-metal interface synergistic effect
A technology of synergy and carbon dioxide, applied in the direction of electrodes, electrolytic components, electrolytic processes, etc., can solve the problems of low efficiency and no selectivity, and achieve the effects of easy adjustment, improved selectivity, and guaranteed catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Synthesis and activation of a three-dimensional covalent organic framework (COF-300) based on imine bonding
[0025] Following the method in the literature [J.Am.Chem.Soc.,2009,131,4570-4571], 100mg of tetraaminophenylmethane and 64mg of terephthalaldehyde were dissolved in 2mL of 1,4-dioxane, and then Add it into the glass tube, and exchange the atmosphere in the glass tube with argon, and seal the tube after freezing in liquid nitrogen; place the glass tube in a 120°C oven for 3 days, then filter the solid, and put the resulting solid in 1,4-dioxane and acetone were used for reflux activation in a Soxhlet extractor, followed by activation with supercritical carbon dioxide to obtain a yellow covalent organic framework material based on imine bonding, namely activated COF -300 powder.
[0026] 2. Preparation of COF-300-AR Based on Amine Bonding
[0027] Add 57.6 mg (1 equivalent) of terephthalic acid and 50 mg (1 equivalent, based on the number of imine bonds) of a...
Embodiment 2
[0028] Example 2: Preparation of COF-metal electrodes
[0029] Put 5 mg of COF-300-AR powder in a mixed solution system formed by 0.75 mL of deionized water, 0.25 mL of ethanol, and 40 μL of 5% Nafion solution, ultrasonically disperse for 5 minutes, then take 100 μL of the dispersion, and slowly drop it onto silver foil (purchased from AlfaAesar) and dried under an infrared lamp to obtain a COF-silver electrode.
Embodiment 3
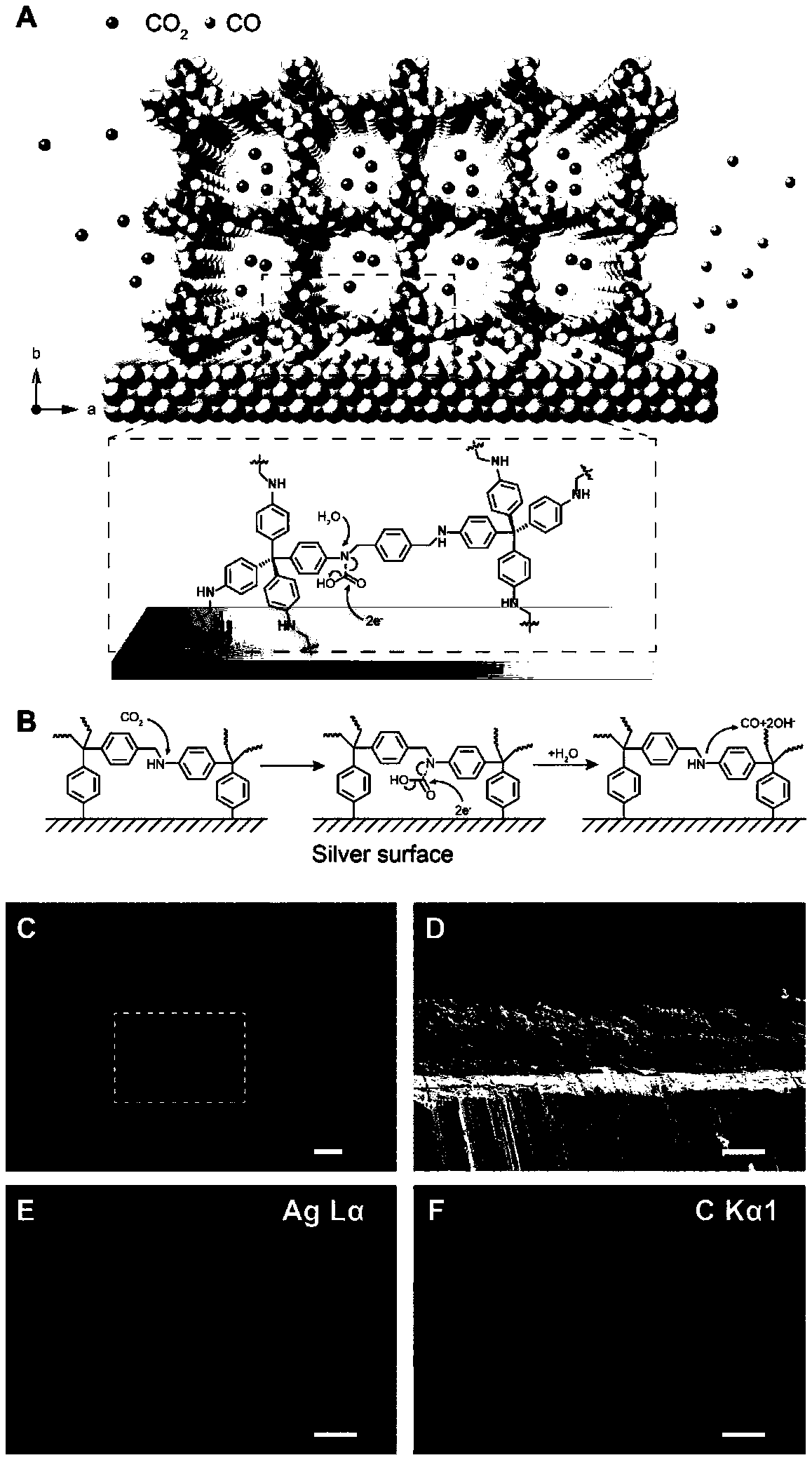
[0031] The COF-metal interface on the COF-silver electrode constructed of COF-300-AR and silver foil was characterized by scanning electron microscope and EDX energy spectrum, as shown in figure 1 As shown in (C-F), it is found that the COF-metal interface is clear and the morphology is relatively uniform. The thickness of the COF-300-AR layer is about 15 microns. The schematic diagram of the interface is shown in figure 1 (A) shown.
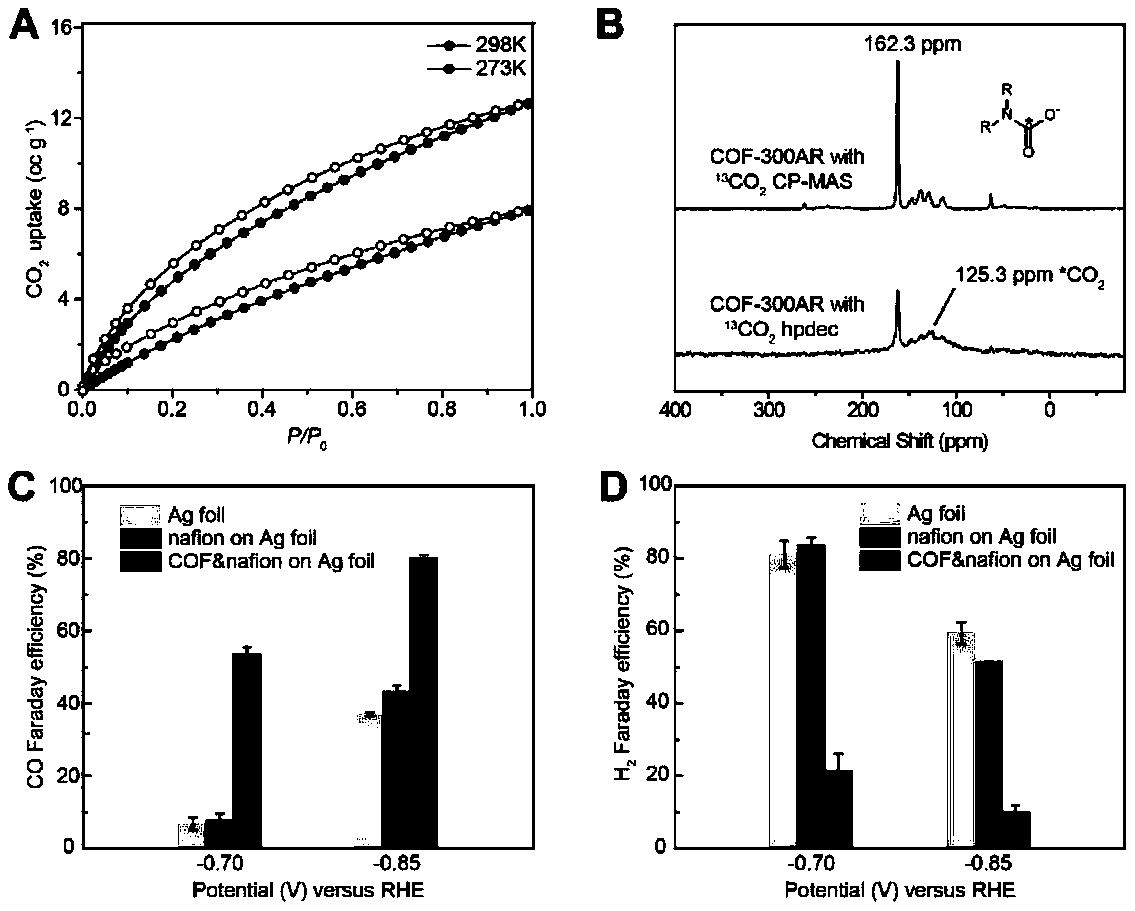
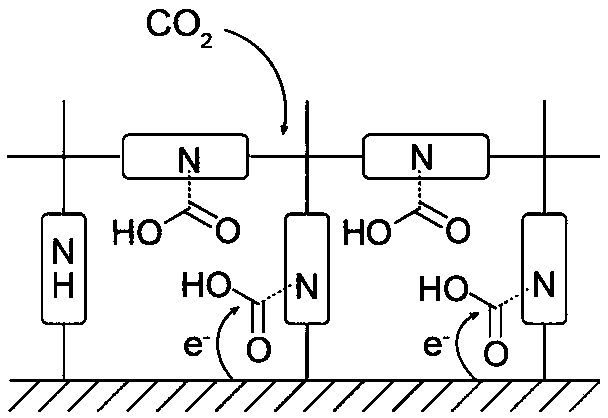
[0032] At the same time, spectrum technology was used to characterize the interaction mode between COF-300-AR and carbon dioxide molecules, and it was found that it contained an amide-like structure, which made us believe that COF-300-AR has the function of enriching and transferring carbon dioxide in the synergistic interface. Further embodies the advantages of this cooperative interface in catalytic reactions.
[0033] The COF-300-AR is interacted with isotope-labeled carbon dioxide, and the mechanism of the COF-metal electrode catalytic syst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com