Method for preparing trans-anethole-albumin nanoparticles with indocyanine green fluorescence tracer
A nano-technology of trans-anethole and albumin, which is applied in the directions of nanotechnology, nanotechnology, nano-medicine, etc., can solve the problems such as research reports of nano-particles without trans-anethole, and achieves reduction in particle size, simple and convenient synthesis process, Small particle size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Preparation of drug-loaded bovine serum albumin nanoparticles: take 2 ml of 10 mg / ml bovine serum albumin aqueous solution, and make it uniform by ultrasonication.
[0040] 2) Slowly add 15 μl of 40 mg / ml trans-anethole solution prepared in dichloromethane and 2 μl of 80 mg / ml indocyanine green solution with a pipette gun.
[0041] 3) After stirring evenly, quickly add 100 μl of glutaraldehyde crosslinking agent aqueous solution, and finally stir and react at 700 rpm / min at 20° C. for 3 hours.
[0042] 4) After the reaction is completed, filter with a 220nm water filter.
[0043] 5) The filtrate was centrifuged at 8,000 rpm / min for 4 minutes, and washed 5 times with deionized water to obtain bovine serum albumin nanoparticles loaded with trans-anethole and indocyanine green.
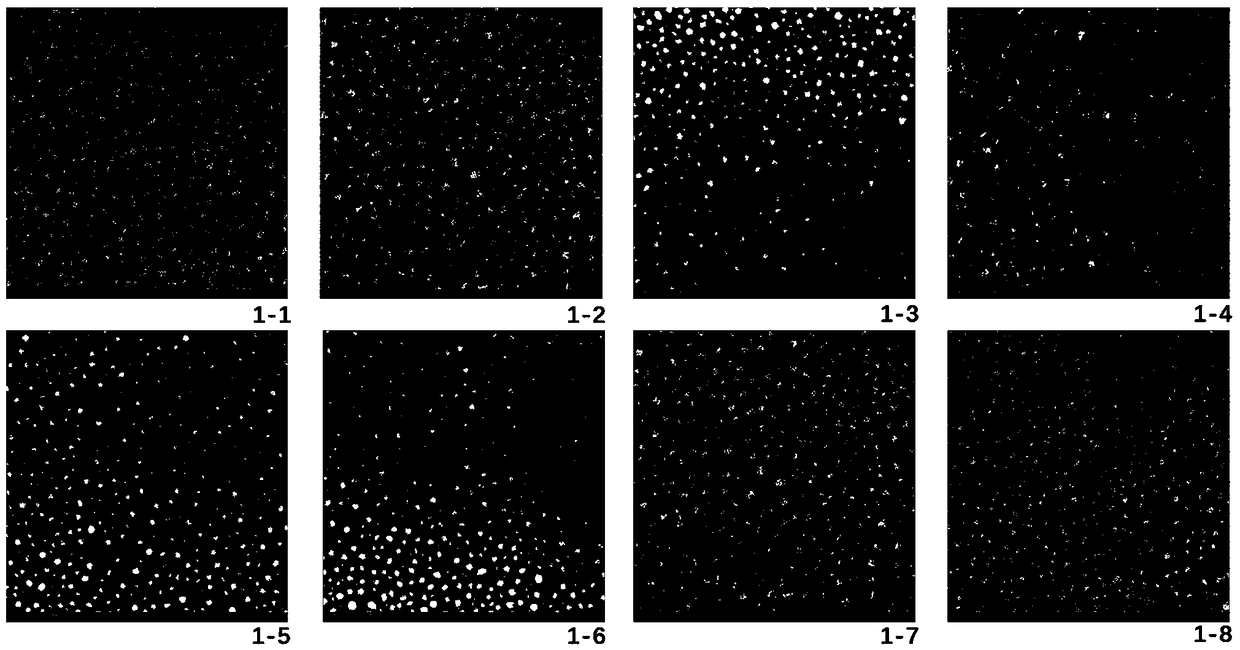
[0044] The morphology of trans-anethole-albumin nanoparticles prepared with indocyanine green fluorescent tracer was observed by transmission electron microscope ( Picture 1-1 ).
Embodiment 2
[0046] 1) Preparation of drug-loaded bovine serum albumin nanoparticles: take 2 ml of 15 mg / ml bovine serum albumin aqueous solution, and make it uniform by ultrasonication.
[0047] 2) Slowly add 12 μl of 40 mg / ml trans-anethole solution prepared in dichloromethane and 1 μl of 80 mg / ml indocyanine green solution with a pipette gun.
[0048] 3) After stirring evenly, quickly add 80 μl of glutaraldehyde crosslinking agent aqueous solution, and finally stir and react at 800 rpm / min at 20° C. for 4 hours.
[0049]4) After the reaction is completed, filter with a 220nm water filter.
[0050] 5) The filtrate was centrifuged at 8,000 rpm / min for 4 minutes, and washed 5 times with deionized water to obtain bovine serum albumin nanoparticles loaded with trans-anethole and indocyanine green.
[0051] The morphology of trans-anethole-albumin nanoparticles prepared with indocyanine green fluorescent tracer was observed by transmission electron microscope ( Figure 1-2 ).
Embodiment 3
[0053] 1) Preparation of drug-loaded bovine serum albumin nanoparticles: take 2 ml of 15 mg / ml bovine serum albumin aqueous solution, and make it uniform by ultrasonication.
[0054] 2) Slowly add 12 μl of a 50 mg / ml trans-anethole solution prepared in dimethyl sulfoxide and 1 μl of a 90 mg / ml indocyanine green solution with a pipette gun.
[0055] 3) After stirring evenly, quickly add 100 μl of glutaraldehyde crosslinking agent aqueous solution, and finally stir and react at 700 rpm / min at 20° C. for 3 hours.
[0056] 4) After the reaction is completed, filter with a 220nm water filter.
[0057] 5) The filtrate was centrifuged at 7,000 rpm / min for 5 minutes, and washed 6 times with deionized water to obtain bovine serum albumin nanoparticles loaded with trans-anethole and indocyanine green.
[0058] The morphology of trans-anethole-albumin nanoparticles prepared with indocyanine green fluorescent tracer was observed by transmission electron microscope ( Figure 1-3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com