High visible light activated graphite-phase carbon nitride nanosheet and preparation method thereof
A graphitic carbon nitride, visible light technology, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., can solve problems such as high energy consumption, affecting carbon nitride polymerization, and time-consuming, and achieve strong visible light absorption performance. , the effect of superior photocatalytic performance and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: A kind of graphitic phase carbon nitride is prepared by the following method:
[0039] Take 12g of dicyandiamide in a 50mL corundum crucible, cover it, put it in a muffle furnace, and put it in a static air atmosphere at 5°C min -1 The temperature was raised to 550°C, and thermal polymerization was carried out at this temperature for 4 hours. After it was cooled to room temperature, the resulting yellow catalyst was taken out and ground into powder, and the obtained sample was marked as S1 sample.
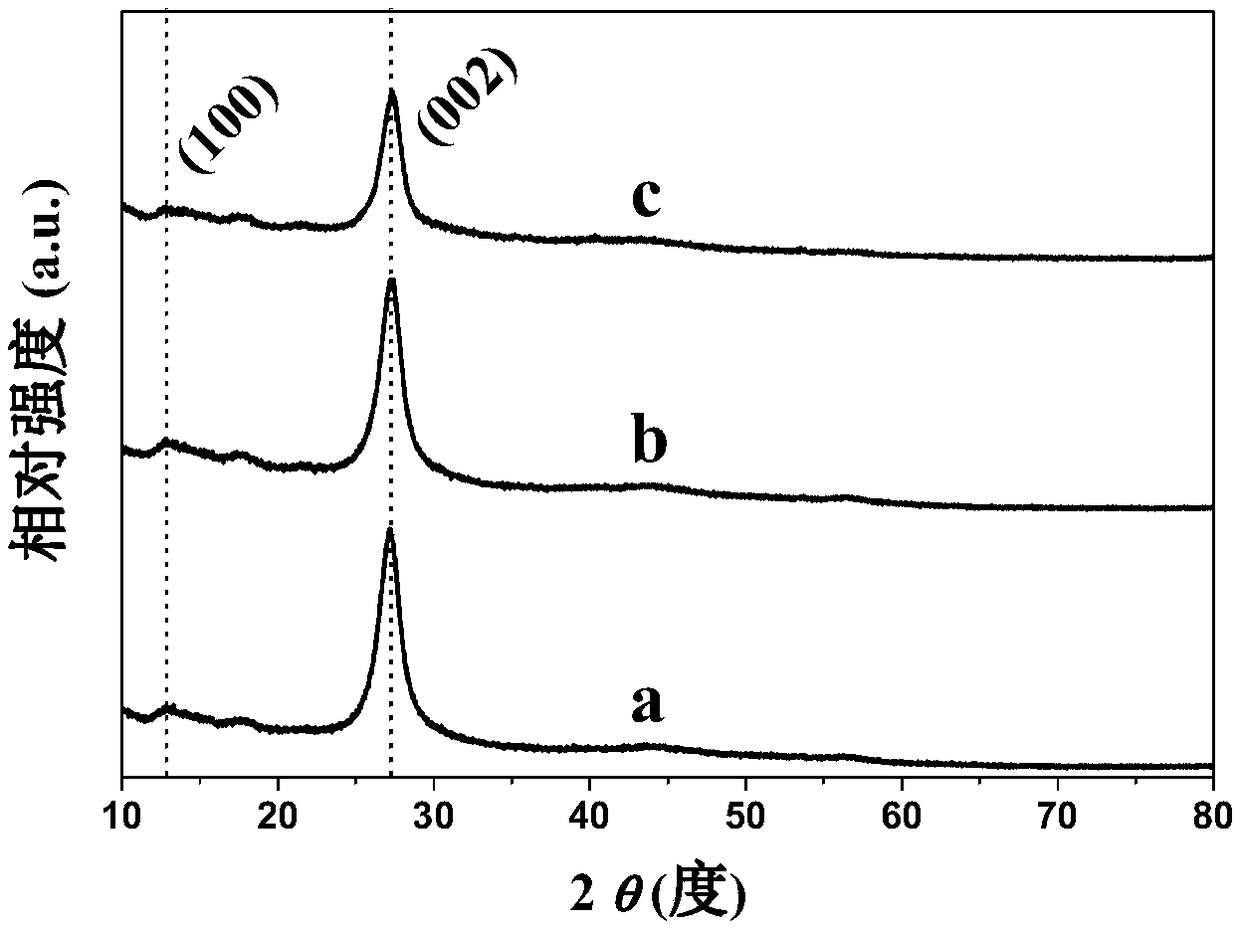
[0040] Such as figure 1 As shown in line a in the middle, the results of the powder X-ray diffraction spectrum show that sample S1 has two characteristic diffraction peaks at 13.1° and 27.4°, corresponding to (100) and (002) characteristic peaks of graphite phase carbon nitride respectively, and reported in the literature The results are consistent with (Adv. Mater. 2014, 26, 8046–8052), indicating that S1 is a graphitic carbon nitride. The Fourier transform...
Embodiment 2
[0042] Embodiment 2: A kind of graphite phase carbon nitride nanosheet with high visible light activity is prepared by the following method:
[0043] Take 1.5g of dicyandiamide and 64mL of water in a 100mL hydrothermal reaction kettle for hydrothermal reaction. After sealing, transfer it to an oven to heat up to 200°C and keep it warm for 1h. After it cools down to room temperature, collect the mixed solution in the kettle. , followed by rotary evaporation to remove water therein to obtain a solid intermediate product.
[0044] Take 12g of the solid intermediate product in a 50mL corundum crucible, cover it, place it in a muffle furnace, and heat it at 5°C min under a static air atmosphere. -1 The temperature was raised to 550°C, and thermal polymerization was carried out at this temperature for 4 hours. After it was cooled to room temperature, the resulting yellow catalyst was taken out and ground into powder, and the obtained sample was marked as S2 sample.
[0045] Such a...
Embodiment 3
[0047] Embodiment 3: A kind of graphite phase carbon nitride nanosheet with high visible light activity is prepared by the following method:
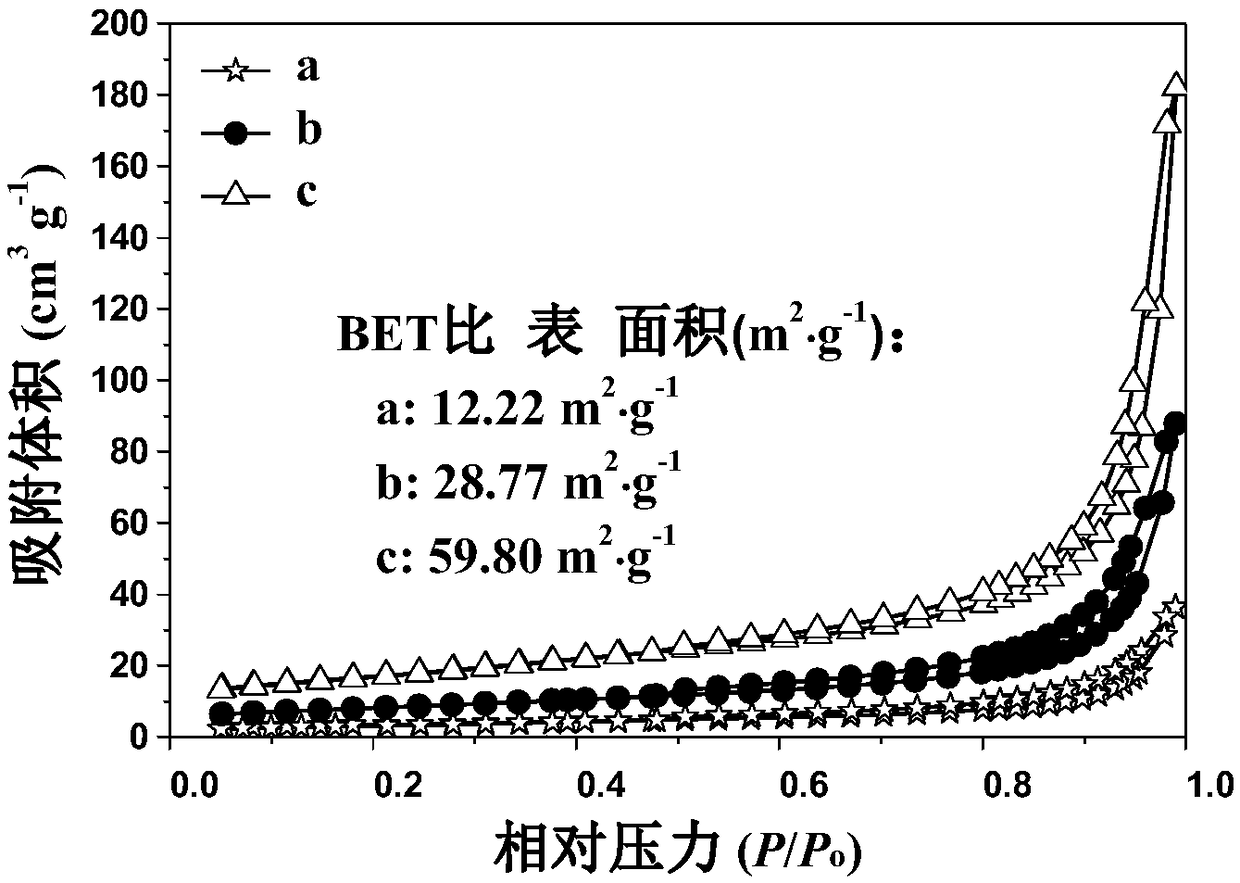
[0048] To examine the effect of hydrothermal pretreatment time on the morphology and photocatalytic performance of the obtained carbon nitride catalysts, we performed hydrothermal time-controlled experiments. Except that the hydrothermal time was adjusted to 4h, other reaction conditions and operations were exactly the same as in Example 2, and the obtained sample was marked as S3 sample.
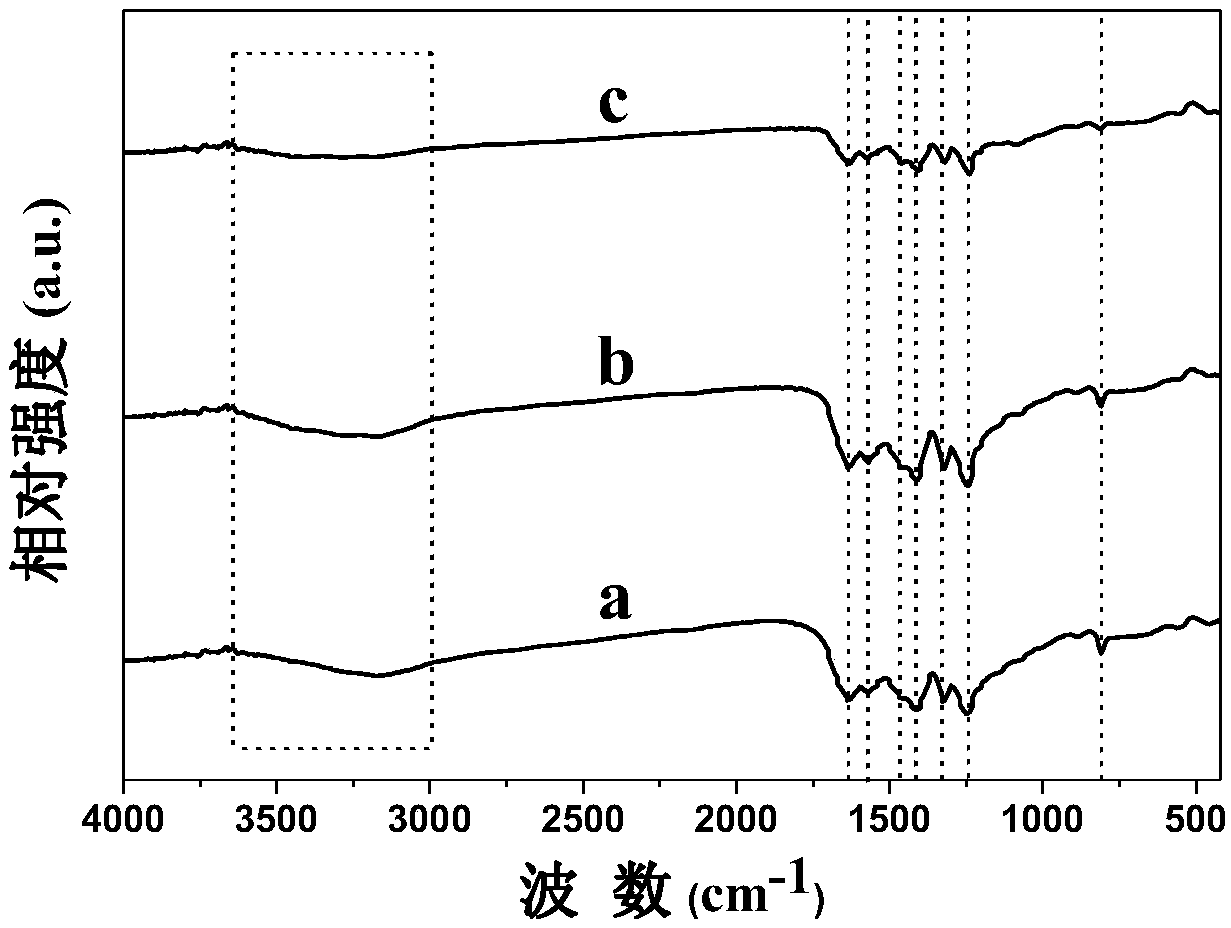
[0049] Such as figure 1 As shown in line c in the middle, the results of the powder X-ray diffraction spectrum show that the sample S3 is graphite phase carbon nitride, but the diffraction peak intensity of S3 at 27.4° is obviously weakened, suggesting that the carbon nitride is stripped. The Fourier transform infrared spectrum of sample S3 as figure 2 As shown in line c in the figure, the peak marked by the dotted line in the figure is the same a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com