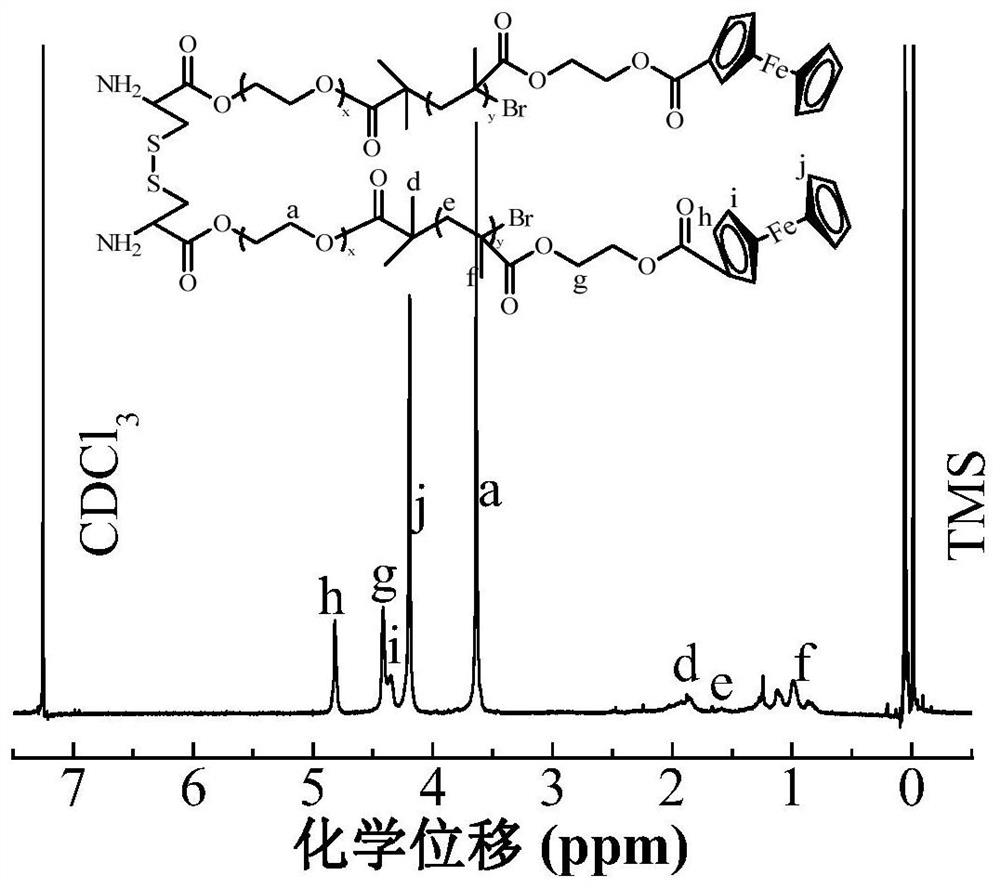
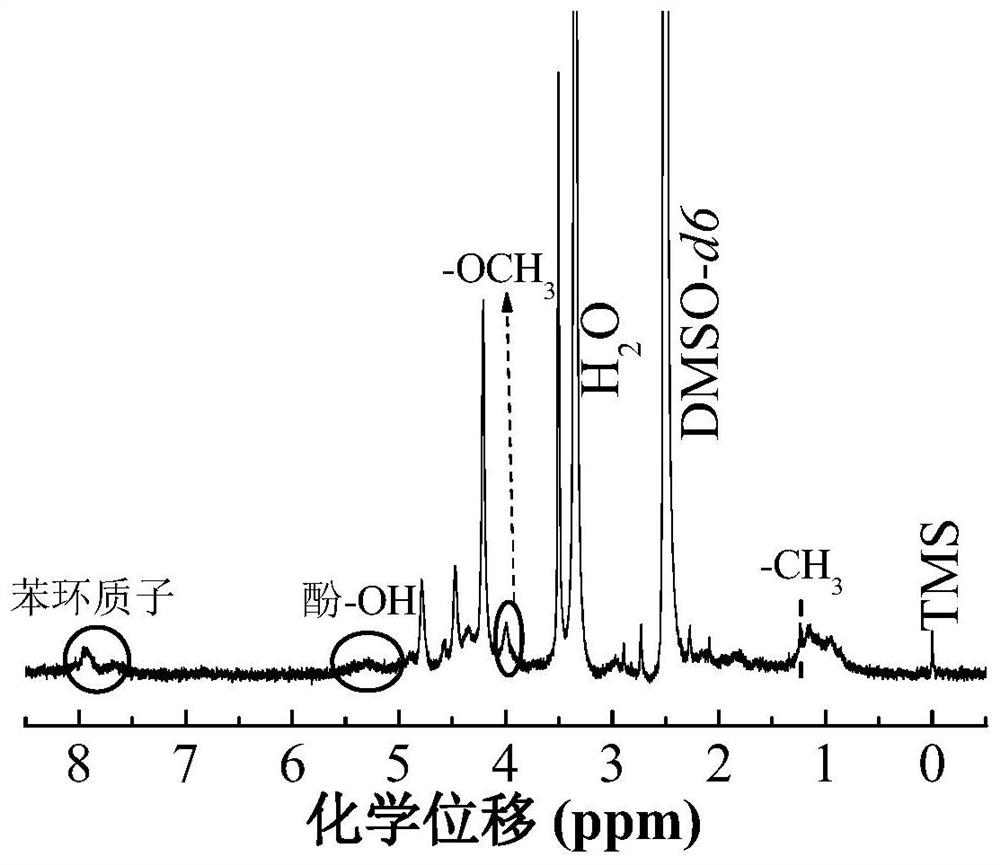
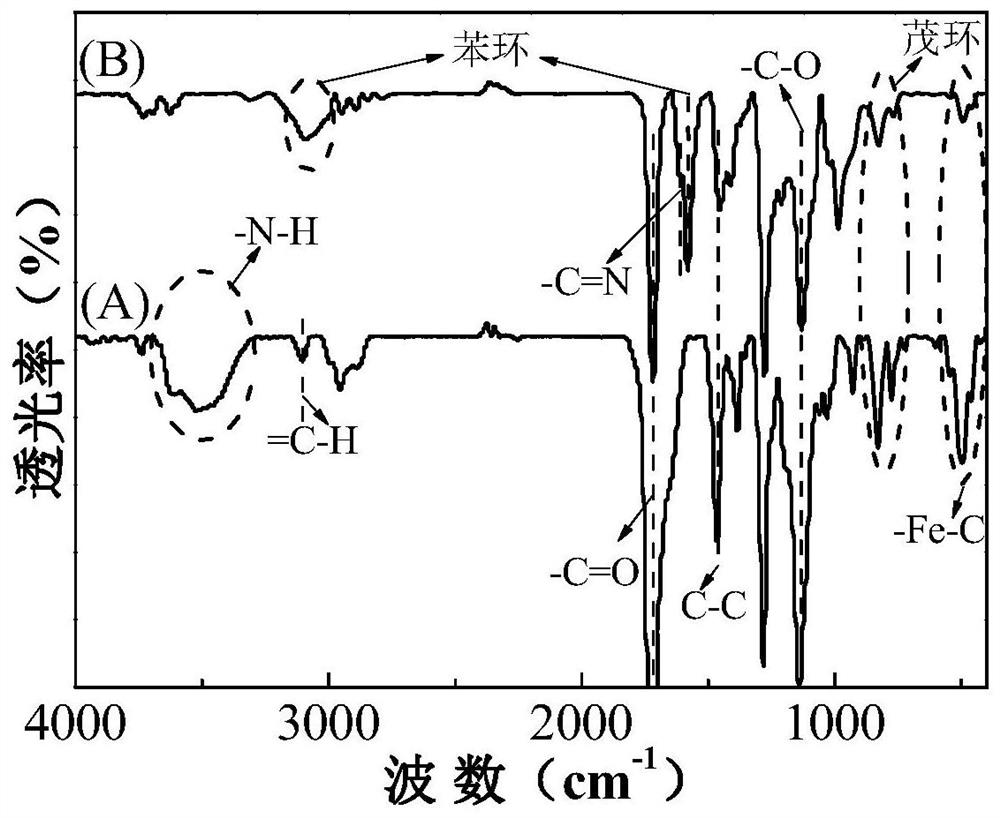
A drug delivery material with pH and double redox responsiveness and its preparation method and application
A responsive, drug technology, applied in the direction of drug delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low drug loading, unsatisfactory drug carrier stability, single drug, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]1, prepare N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester)2
[0046]0.30 g (0.68 mmol) of N, N'-bis (tert-butoxycarbonyl) -L-cystine (according to the literature "Hyun-chul Kim, Eunjoo Kim, Tae-Lin Ha, Sang Won Jeong , Se Guen Lee, Sung Jun Lee, BoramLee.Thiol-responsive Gemini poly (ethylene glycol) -poly (lactide) with a cystinedisulfide spacer as an intracellular drug delivery nanocarrier.Colloids andSurfaces B: Biointerfaces, 2015,127,206-212 "disclosed in Methods were prepared by 3.26 g (1.63 mmol) polyethylene glycol 2000 dissolved in 50 ml of anhydrous dichloromethane and drumped into nitrogen, add 0.42 g (2.05 mmol) N, N'-bicyclic hexyl carbon diimide back room temperature. The reaction was reacted for 48 hours. After the end, the insoluble matter was removed, and the filtration was evaporated to remove the solvent, precipitated in excess diethyl ether, and the precipitate vacuum was dried to constant weight, and the crude product was reinurated in ...
Embodiment 2
[0063]1, prepare N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester)2
[0064]This step is the same as step 1 of Embodiment 1.
[0065]2. Preparation of macromolecular initiators
[0066]This step is the same as step 2 of Example 1.
[0067]3, prepare N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester)2-B- (polymethyl methacrylate ferrousoxyeta)2Block polymer
[0068]0.30g (6.38 × 10)-2Mmol) Macromolecular initiator and 2.64 g (7.66 mmol) of methacrylate in 5 ml of anhydrous o, N-dimethylformamide, added under nitrogen protection 79.50 μL (0.383 mmol) 5 methylxide triamine and 31.60 mm (0.319 mmol) chloride were subsequently "frozen - evacuated - thaw" operation three times, and the polymerization was carried out at 90 ° C for 48 hours. After the reaction is completed, the reaction liquid passes through a neutral alumina column, and the resulting liquid is spurred and then precipitated three times in excess diethyl ether, and the vacuum is dried to constant we...
Embodiment 3
[0077]1, prepare N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester)2
[0078]0.30 g (0.68 mmol) of N, N'-bis (tert-butoxycarbonyl) -L-cystine and 6.52 g (1.63 mmol) polyethylene glycol 4000 were dissolved in 75 ml anhydrous dichloromethane. The nitrogen was drumped, 0.42 g (2.05 mmol) N, N'-bicyclic hexyl carbon diamine reaction was added at 48 hours. After the end, the insoluble matter was removed, and the filtration was evaporated to remove the solvent, precipitated in excess diethyl ether, and the precipitate vacuum was dried to constant weight, and the crude product was reinurated in water into the dialysis bag of the trapped molecular weight of 3500. Dialysis , Freeze dry to obtain N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester) as shown in Formula II-32The yield is 28%.
[0079]
[0080]2. Preparation of macromolecular initiators
[0081]1.00 g (0.119 mmol) of N, N'-bis (tert-butoxycarbonyl) -L-cystine-(polyethylene glycol ester) of formula II-32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com