Benzene-terthienyl-benzene derivative as well as preparation method and application thereof
A technology of triple thiophene and benzene derivatives, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as poor solubility and high rigidity of the main chain, and achieve improved stability, fast response speed, and increased total The effect of the degree of yoke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Specific Synthesis of 2-(1,3,5-tris(2-thiophene)benzene)[4,4,5,5-tetramethyl-1,3,2-dioxaborane] Compound
[0025] 1-(2-(5-bromothiophene))-3,5-bis(2-thiophene)benzene (1.62g, 4.0mmol), bis(pinacolate)diboron (2.03g, 8.0mmol), KOAc (0.79g, 8.8mmol) and Pd(PPh 3 )Cl 2 (10.0mg, 0.007mmol) was dissolved in 60mL of dioxane under the protection of nitrogen, and the temperature was rapidly raised to 130°C, the system was refluxed for 24h, the reaction was stopped, cooled to room temperature, extracted with deionized water and dichloromethane, and the obtained The organic phase was dried by adding anhydrous MgSO4, and then separated and purified by column chromatography. The stationary phase was 300 mesh silica gel, and the mobile phase was dichloromethane / petroleum ether (1:3). Finally, a green solid 2-(1,3 ,5-tris(2-thiophene)benzene)[4,4,5,5-tetramethyl-1,3,2-dioxaborane] 1.64g, the yield was 91%. 1 H NMR (500MHz, CDCl 3 )δ7.74(dd, J=6.0,1.5Hz,3H),7.36(d,J=3.7Hz,2H),7.27...
Embodiment 2
[0027] Preparation of Benzene-Terthiophene-Benzene Derivatives
[0028] 1-(2-(5-bromothiophene))-3,5-bis(2-thiophene)benzene (0.24g, 1.0mmol), 2-(1,3,5-tri(2-thiophene)benzene)[ 4,4,5,5-tetramethyl-1,3,2-dioxaborane] (0.90g, 2.0mmol), K 2 CO 3 (1.1g, 8mmol), 4ml water, Pd(PPh 3 ) 4 (10.0mg, 0.007mmol) was added sequentially under a nitrogen atmosphere, 6ml of toluene was added, the temperature was rapidly raised to 130°C, the system was refluxed for 24h, the reaction was stopped, cooled to room temperature, extracted with deionized water and dichloromethane, and the obtained organic phase was added to After drying with anhydrous MgSO4, separate and purify by column chromatography, the stationary phase is 300 mesh silica gel, the mobile phase is dichloromethane / petroleum ether (1:5), and finally a yellow solid benzene-terthiophene-benzene derivative 1.15 g, the yield is 75%.MALDI-TOF-MS(M)(m / z):647.5[M+H] + . 1 H NMR (400MHz, CDCl 3 )δ7.77(s,6H),7.47–7.45(dd,4H),7.39(t,J...
Embodiment 3
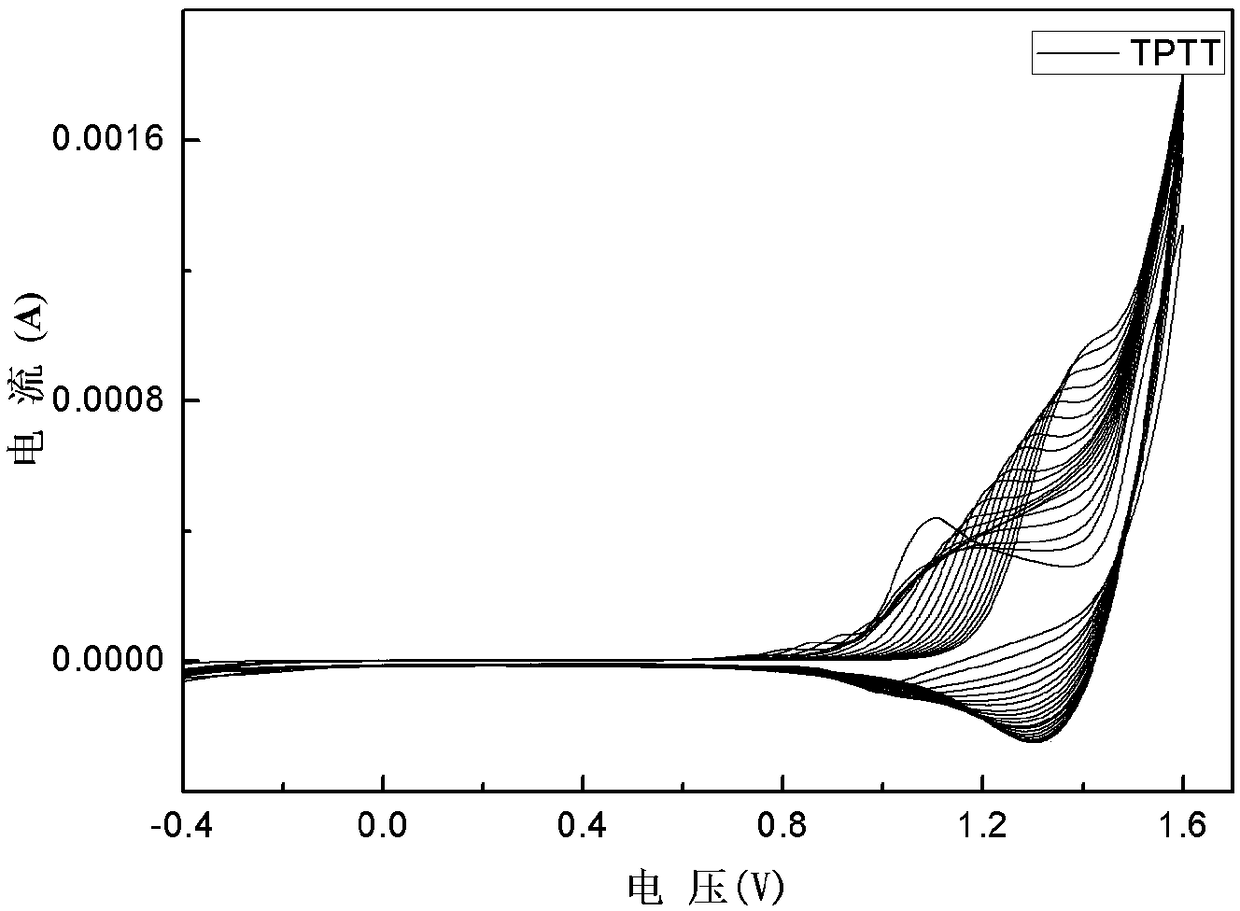
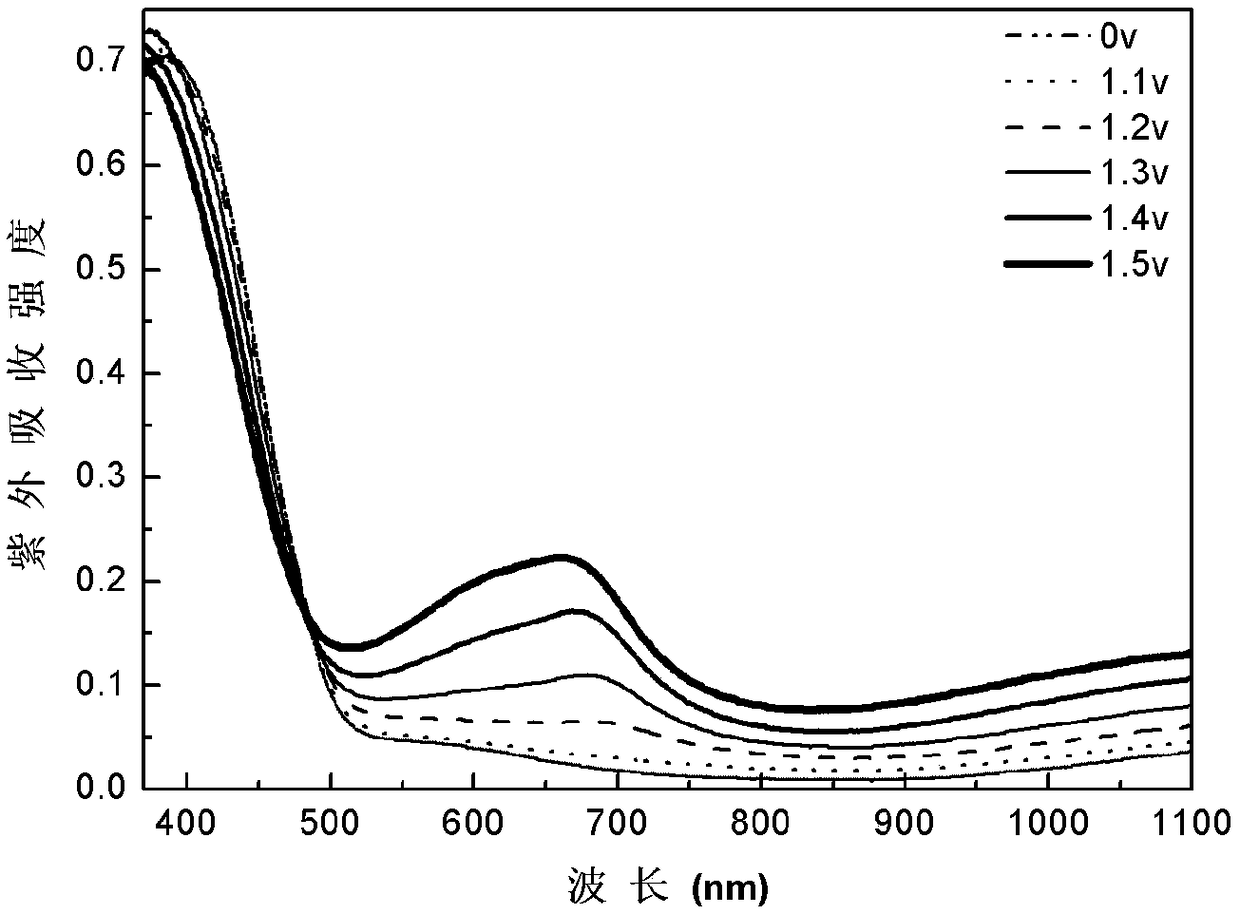
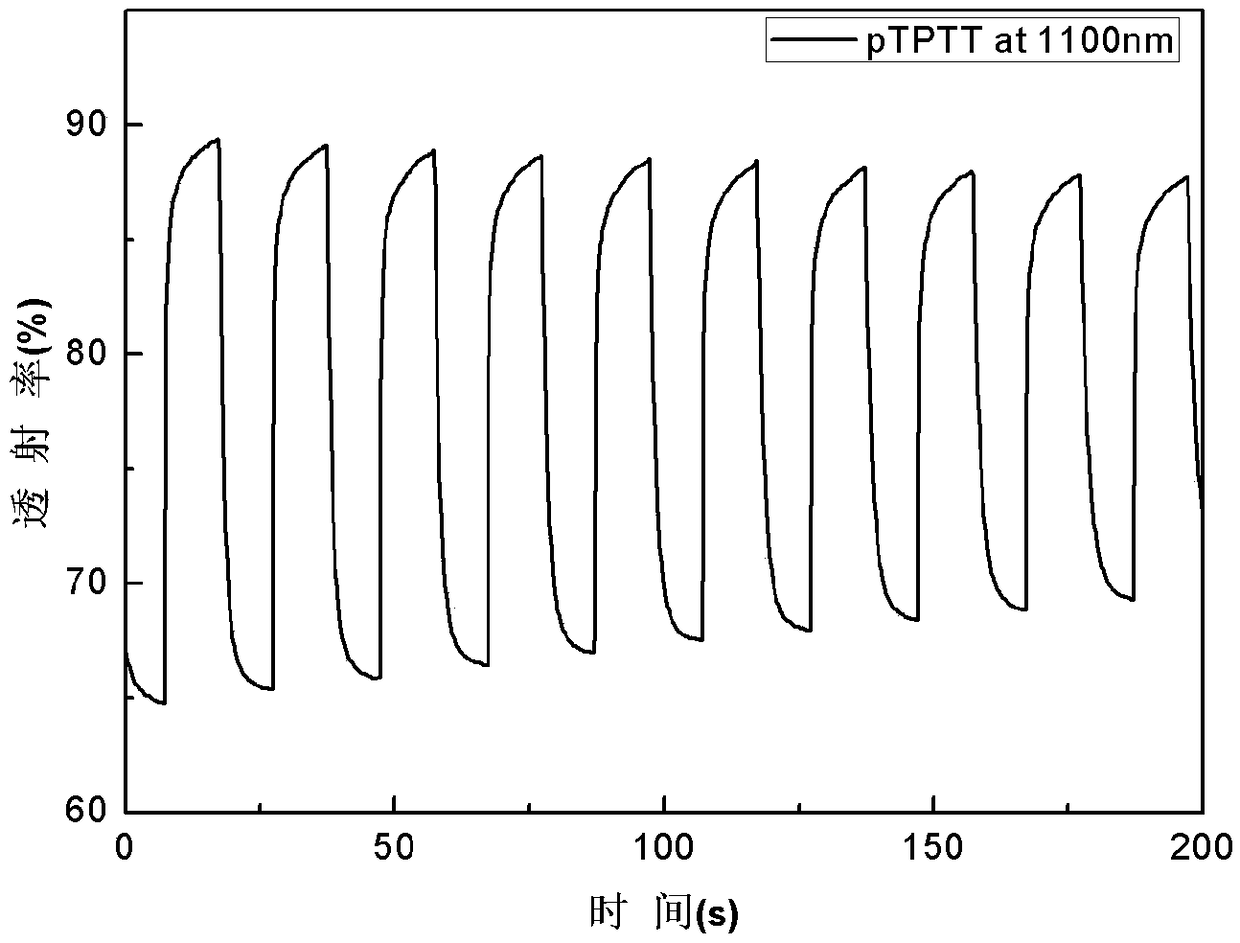
[0030] Dissolve benzene-terthiophene-benzene derivatives in dichloromethane / acetonitrile solution (volume ratio 7:3), tetrabutylammonium hexafluorophosphate (TPAPF 6) as the electrolyte, constant volume, ultrasonic 3min, until completely dissolved, the electrode is platinum wire as the counter electrode, Ag / AgCl electrode as the reference electrode, and ITO conductive glass as the three-electrode system of the working electrode, using cyclic voltammetry 0- 1.6V electrochemical polymerization film formation, the polymerization conditions are: the voltage range is 0-1.6v, the scanning speed is 0.1v / s, and the number of scanning circles is 20 circles. in TPAPF 6 / dichloromethane / acetonitrile (v:v,7:3) for 1min dedoping for performance testing, all electrochemical tests were performed at TPAPF 6 / dichloromethane / acetonitrile (v:v, 7:3) for testing. Cyclic voltammetry, spectroelectrochemical and electrochromic test step voltage is 0V-1.6V, attached figure 1 , 2 , 3, 4 are the C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com