Eye drops containing rupatadine fumarate and preparation method thereof
A technology of rupatadine fumarate and eye drops, which is applied in the field of medicine, can solve problems such as limited curative effect, and achieve the effects of small toxic and side effects, convenient industrial production, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The present invention also provides a preparation method of the eye drops, which can adopt various existing preparation techniques for preparing eye drops; the present invention also provides the preparation method of the eye drops, comprising the following steps: taking an appropriate amount of water for injection to dissolve rupatadine acid and Tribulus terrestris extract to obtain solution A; then take an appropriate amount of water for injection to dissolve the eye drop auxiliary materials to obtain solution B; then mix solution A and solution B, and add the rest of the water for injection to obtain the product.
[0021] The present invention also provides a preparation method when the eye drops contain a thickener, which includes the following steps: take an appropriate amount of water for injection to dissolve rupatadine fumarate and Tribulus terrestris extract to obtain solution A; then take an appropriate amount for injection Dissolve the auxiliary materials of t...
Embodiment 1-3
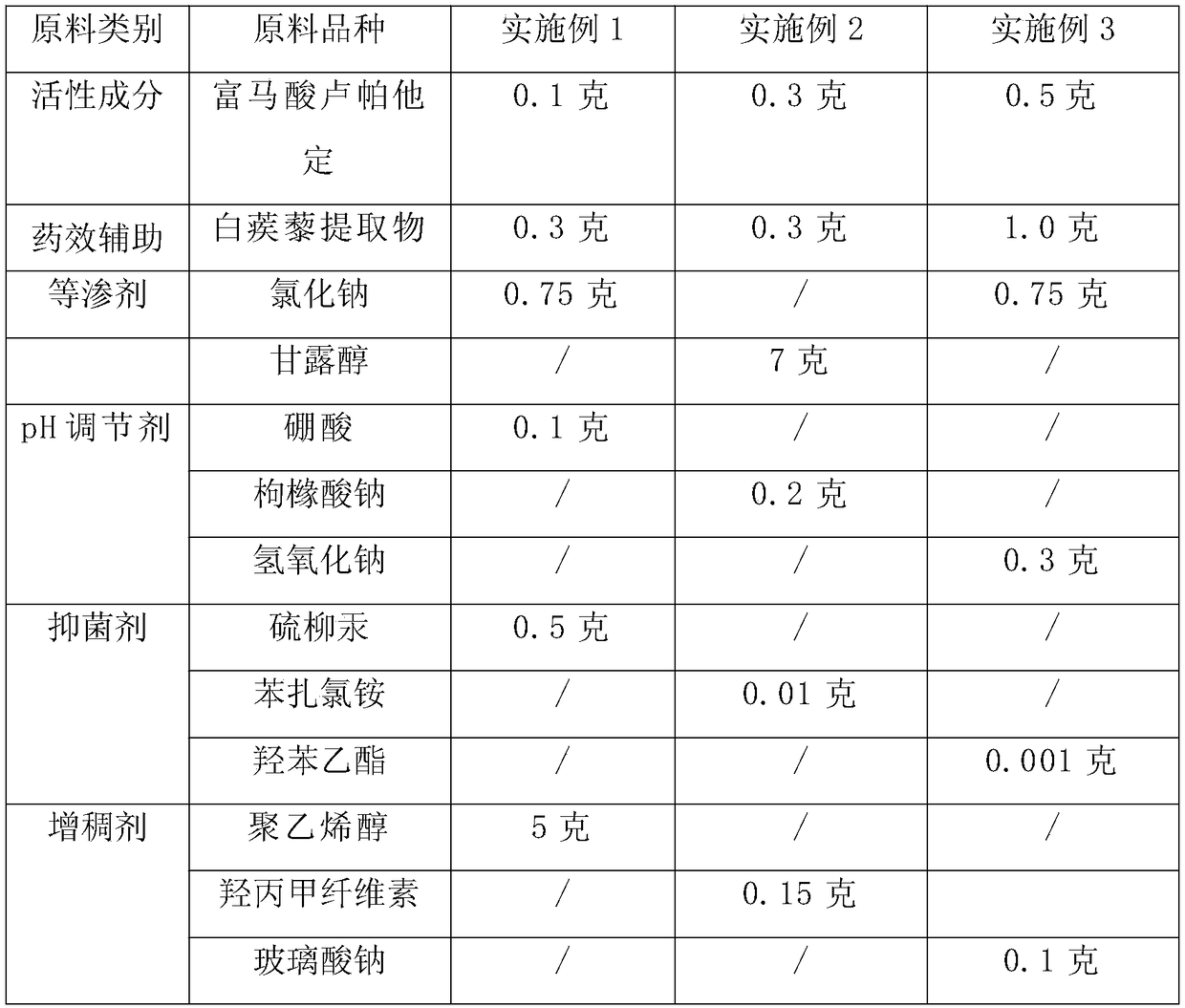
[0026] Table 1: Formula table of embodiment 1-3 eye drops
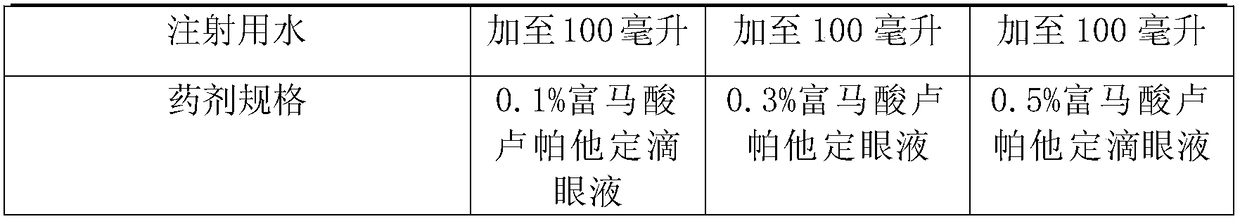
[0027]
[0028]
[0029] According to the technical scheme of the present invention, the adjuvant species that can be selected for preparing eye drops containing rupatadine fumarate are not limited to the species listed in the above table, and the following multiple options can also be selected:
[0030] As bacteriostatic agent, any bacteriostatic agent called in pharmacy can be used, and its consumption is according to the conventional dosage in pharmacy. For example, ① 0.002%-0.005% thimerosal; ② quaternary ammonium salts (including benzalkonium chloride, benzalkonium bromide), dumiphene, spirityl, etc., the effective concentration is 0.002%-0.01%; ③ alcohols, commonly used 0.3- 0.6% chlorobutanol; ④ Parabens, commonly used 0.03-0.06% ethylparaben; ⑤ Acids, such as 0.01-0.08% tricolic acid. The concentrations of the above substances are all volume-weight percentages, that is, every 100 milliliters contains gr...
experiment example 1
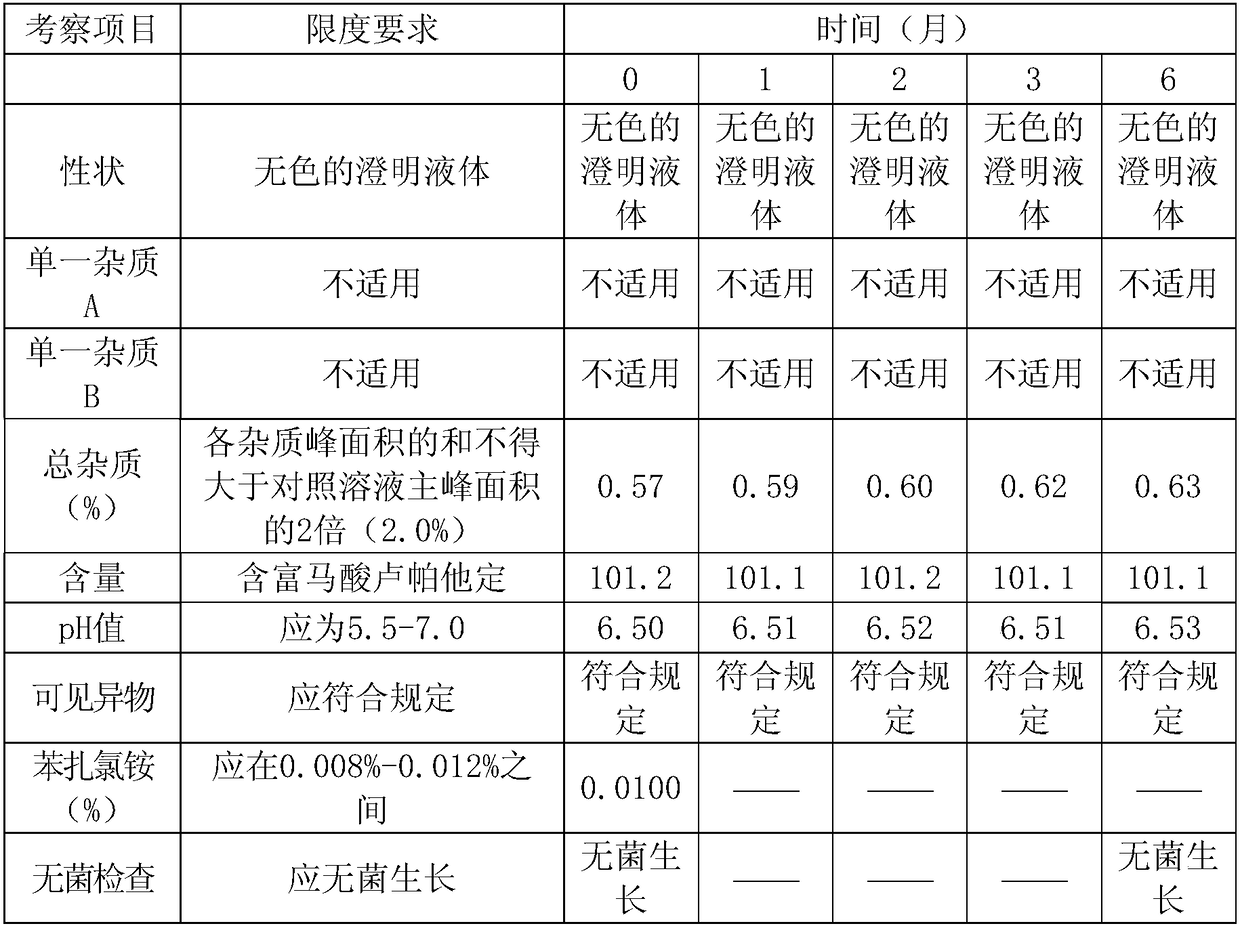
[0034] Stability test of experimental example 1 rupatadine fumarate eye drops
[0035] Accelerated test: the 0.3% rupatadine fumarate eye drops prepared by the method of Example 2 of the present invention was put into a constant temperature and humidity box under the commercially available packaging conditions, at a temperature of 40°C ± 2°C and a relative humidity of 25°C. %±5%, samples were taken on time at the 1st, 2nd, 3rd, and 6th months respectively, and measured according to the relevant requirements of the Chinese Pharmacopoeia 2015 edition. The results are shown in Table 1:
[0036] Table 1: Stability test data table
[0037]
[0038] Long-term test: 0.3% rupatadine fumarate eye drops prepared according to the method of Example 2 of the present invention was put into a constant temperature and humidity box under the commercially available packaging conditions, and the temperature was 25°C ± 2°C, and the relative humidity was 40°C. % ± 5%, samples were taken on time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com