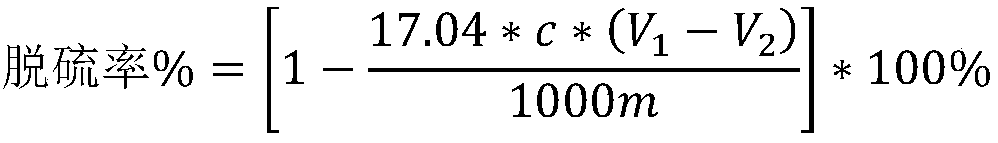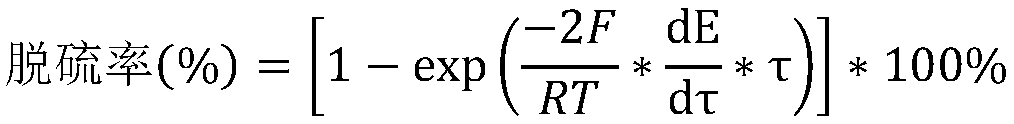Method for oxidatively removing hydrogen sulfide
A hydrogen sulfide and removal technology, applied in the field of oxidative removal of hydrogen sulfide and hydrogen sulfide treatment, can solve the problems of inapplicability of high-concentration pollutants or gaseous pollutants, the reaction is limited by pH, and the equipment investment is large, so as to reduce construction costs. Investment, uncomplicated structure, and the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Reagent preparation: 200g / L sodium hydroxide solution (CAS: 1310-73-2, analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.), 1M hydrochloric acid solution (CAS: 7647-01-0, analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.), 5g / L zinc acetate solution (CAS: 557-34-6, analytically pure, Shanghai Titan Technology Co., Ltd.), 50g / L iodine stock solution (CAS: 7553-56-2, analytically pure, Shanghai Titan Technology Co., Ltd.), 5g / L iodine solution, 0.01M sodium thiosulfate standard titration solution (CAS: 10102-17-7, analytical grade, Shanghai Lingfeng Chemical Reagent Co., Ltd.), 5g / L starch indicator solution ( CAS: 9005-25-8, analytically pure, Shanghai Titan Technology Co., Ltd.). The above reagents are prepared according to the standard in GB / T11060.1-1998.
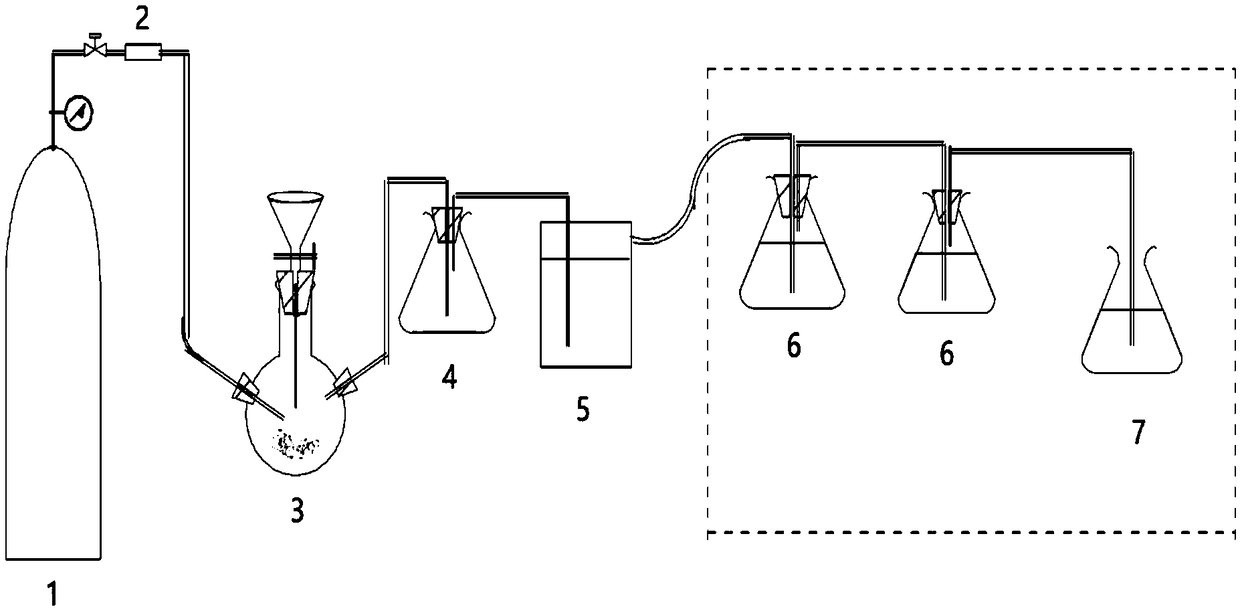
[0027]The experimental device is shown in the figure to simulate the treatment of waste gas containing hydrogen sulfide. There is 2.4 mg of sodium sulfide nonahydrate in the three-necke...
example 2
[0032] The present invention adopts sodium sulfide nonahydrate (CAS: 131-84-4, analytically pure, Shanghai Titan Technology Co., Ltd.) Analytical grade, Shanghai Titan Technology Co., Ltd.)-borax (CAS: 1303-96-4, analytical grade, Shanghai Titan Technology Co., Ltd.) buffer was adjusted to the required pH value. The concentration of sodium sulfide nonahydrate solution is 10 -4 M. Sodium bicarbonate (CAS: 144-55-8, analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.) solution was used as the activator. Add 0.1M sodium chloride (CAS: 7647-14-5, analytical grade, Sinopharm Chemical Reagent Co., Ltd.) to maintain the ionic strength of the solution. Add EDTA (CAS: 6381-92-6, analytically pure, Shanghai Lingfeng Chemical Reagent Co., Ltd.) to reduce metal ion to hydrogen peroxide ((CAS: 7722-84-1, purity=30wt%, Sinopharm Group Chemical Reagent Co., Ltd.) ) catalytic effect.
[0033] The silver / sulfur ion selective electrode and C(K2SO4)-1 reference electrode produced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com