Preparation method for double-layer carbon coated metal sulfide composite electrode material with core-shell structure
A technology of metal sulfide and core-shell structure, applied in nanotechnology for materials and surface science, battery electrodes, structural parts, etc., can solve problems affecting material capacity and performance, increasing irreversible capacity, shortening transmission path, etc. Achieve the effect of improving high rate performance, improving cycle stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add cobalt chloride hexahydrate dihydrate and pyrrole monomer into the reaction vessel at a molar ratio of 1:1, stir and mix evenly; slowly add the prepared ammonium persulfate into the step mixed solution at room temperature to initiate the polymer The monomer was polymerized and maintained for 16 h; the resulting mixture was directly kept in a blast oven at 100 °C for 10 h to obtain polypyrrole-coated Co 9 S 8 Precursor; polypyrrole-coated metal Co 9 S 8 The precursor was placed in a tube furnace, and ethanol was evenly loaded into the furnace with protective gas as the carrier gas. The temperature of the furnace was controlled at 800°C, and the catalytic decomposition reaction of ethanol was carried out on the surface of the precursor. After 5 hours, a coating layer with a thickness of about 8nm core-shell structure double-layer carbon-coated Co 9 S 8 composite material.
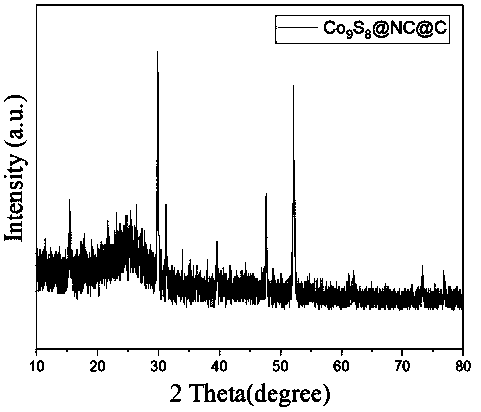
[0031] The prepared composite electrode material is subjected to XRD diffraction test, and...
Embodiment 2
[0034] The influence of CVD on the electrochemical performance during the preparation of composite electrode materials was compared.
[0035] Add cobalt chloride hexahydrate dihydrate and pyrrole monomer into the reaction vessel at a molar ratio of 1:1, stir and mix evenly; slowly add the prepared ammonium persulfate into the mixed solution at room temperature to trigger polymer monomer Bulk polymerization and maintaining the reaction for 16h; the resulting mixture was directly kept in a blast oven at 100°C for 10h to obtain polypyrrole-coated Co 9 S 8 Precursor; polypyrrole-coated metal Co 9 S 8 The precursors were placed in a tube furnace with N 2 For the protective gas, the furnace temperature was controlled at 800 °C, and after 5 h, a single-layer carbon-coated Co 9 S 8 composite material.
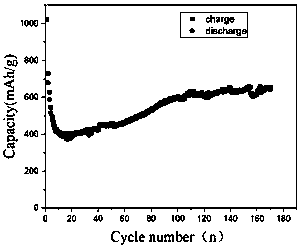
[0036] The obtained composite electrode material was made into a CR2032 button battery, and its charge and discharge performance was tested with a blue electric battery test syst...
Embodiment 3
[0038] The influence of carbon shell thickness on the electrochemical performance during the preparation of composite electrode materials was compared.
[0039] Add cobalt chloride hexahydrate dihydrate and pyrrole monomer into the reaction vessel at a molar ratio of 1:1, stir and mix evenly; slowly add the prepared ammonium persulfate into the mixed solution at room temperature to trigger polymer monomer Bulk polymerization and maintaining the reaction for 16h; the resulting mixture was directly kept in a blast oven at 100°C for 10h to obtain polypyrrole-coated Co 9 S 8 Precursor; polypyrrole-coated metal Co 9 S 8 The precursor was placed in a tube furnace, and ethanol was evenly loaded into the furnace with protective gas as the carrier gas. The temperature of the furnace was controlled at 800°C, and the catalytic decomposition reaction of ethanol was carried out on the surface of the precursor. After 4 hours, a coating layer with a thickness of about 5nm core-shell struc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com