Pleuromutilin compound and preparation method and application thereof
A technology of pleuromutilin and compounds, which is applied in the field of drug synthesis, can solve the problems of few candidate drugs or veterinary drugs, short half-life, poor water solubility, etc., achieve great economic and social benefits, and have little corrosion of equipment and strong The effect of antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
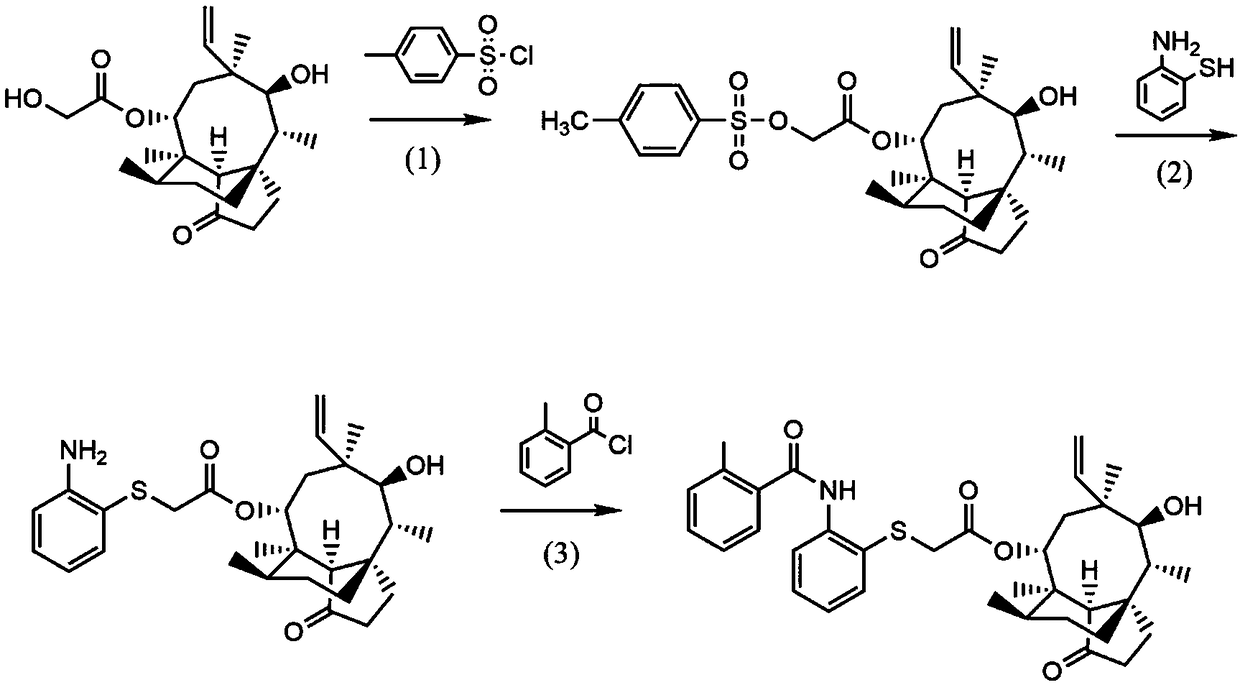
[0065] Example 1 Preparation of p-tosylated pleuromutilin
[0066] (1) Dissolve pleuromutilin (10g, 26.46mmol) in 20mL ethyl acetate to obtain a pleuromutilin solution, then add p-toluenesulfonyl chloride (5.5g, 28.94mmol) to obtain a mixed solution; , add dropwise a sodium hydroxide solution with a concentration of 20 mol / L to the mixed solution until the pH of the system is 12.5, then remove the ice bath, react for 3 hours at 25°C, and the reaction is complete;
[0067] (2) Pour the reaction solution into a separatory funnel, first add 50 mL of chloroform for layering, remove the water phase, and finally wash the organic phase with 100 mL of water twice and dry with anhydrous sodium sulfate;
[0068] (3) Dry the organic phase by rotary evaporation, add 10mL isopropanol to the residual solid and heat to dissolve. After cooling, a large amount of white powder is precipitated, filtered under reduced pressure, and the filtrate is washed with isopropanol, and evaporated to drynes...
Embodiment 2
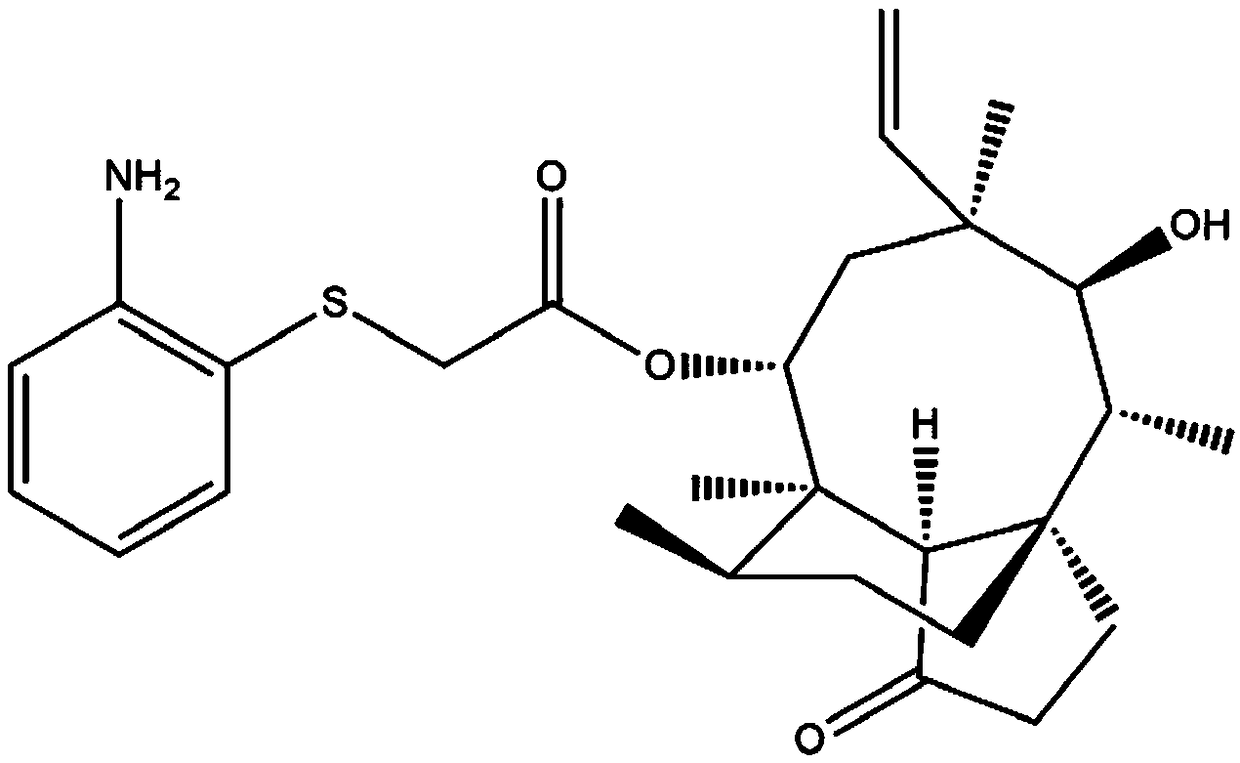
[0069] The preparation of embodiment 2 intermediate (I)
[0070] (1) Under nitrogen protection, add p-tosylated pleuromutilin (1g, 1.88mmol) and ethyl acetate (18.3mL, 188mmol) prepared in Example 1 into a three-necked flask equipped with a thermometer and a stirrer ), dissolved completely at room temperature;
[0071] (2) Add diaminobenzenethiol (0.31g, 2.44mmol) to 10mL of 10wt% sodium hydroxide solution cooled to 0°C to obtain a mixed solution of alkali solution and diaminobenzenethiol; then add the mixed solution dropwise To the reaction flask in step (1), react at 26°C for 3h; finally add TEBAC (0.09g, 0.376mmol), and heat at reflux at 75°C for 3h;
[0072] (3) After the reaction, extract the oil phase with dichloromethane, wash the organic phase with chloroform, saturated brine, and water successively, dry the ethyl acetate with anhydrous sodium sulfate, and obtain the crude product of intermediate (I);
[0073] (4) After dissolving the crude product of the above-menti...
Embodiment 3
[0074] The preparation of embodiment 3 pleuromutilin compounds
[0075] (1) Add o-toluic acid (0.34g, 2.47mmol) and dichloromethane (12.0mL, 188mmol) into a three-neck flask equipped with a thermometer and a stirrer, and dissolve completely at room temperature;
[0076] (2) Take 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (0.47g, 2.47mmol), 1-hydroxybenzotriazole (0.33g, 2.47mmol), Triethylamine (0.86mL, 6.19mmol) was added to the reaction flask, and reacted at 25°C for 5h; the intermediate (I) (1g, 2.06mmol) prepared in Example 2 was added to the reaction flask, and reacted at 25°C for 5h;
[0077] (3) After the reaction, the oil phase was separated with dichloromethane, washed with saturated brine and water, dried with phosphorus pentoxide, and then evaporated to dryness to obtain the crude product of pleuromutilin compounds;
[0078] (4) Dissolve the crude product of the above-mentioned pleuromutilin compound in methanol, add silica gel powder (200-300 mesh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com