Preparation method of chitosan-g-antibacterial peptide polymer
A technology of chitosan and antibacterial peptide is applied in the field of preparation of antibacterial biological materials, which can solve the problems of limited practical application, high cytotoxicity and high production cost, and achieves easy mass production, low bactericidal concentration and low bacteriostatic concentration. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
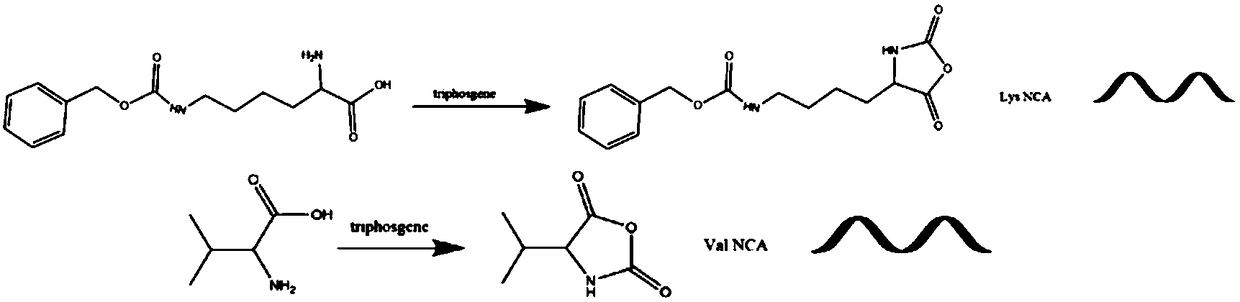
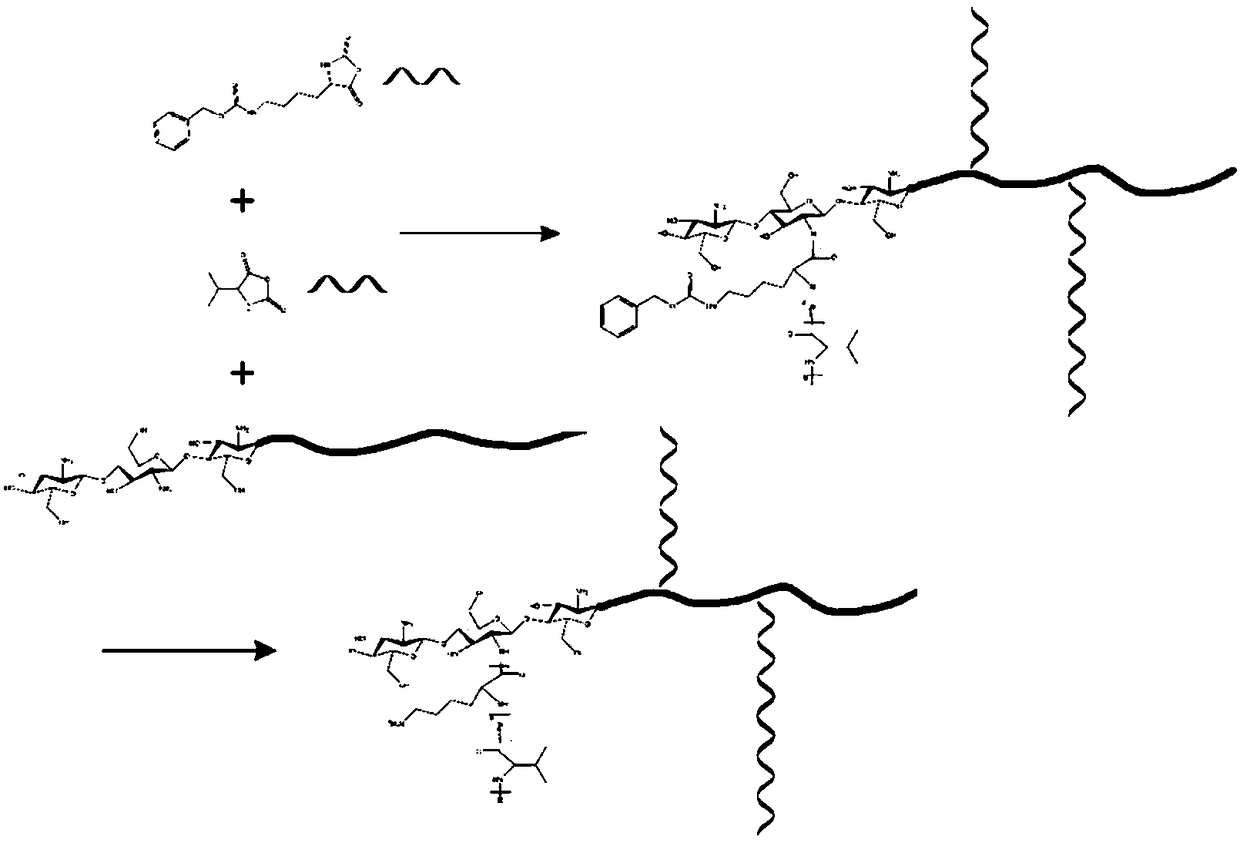
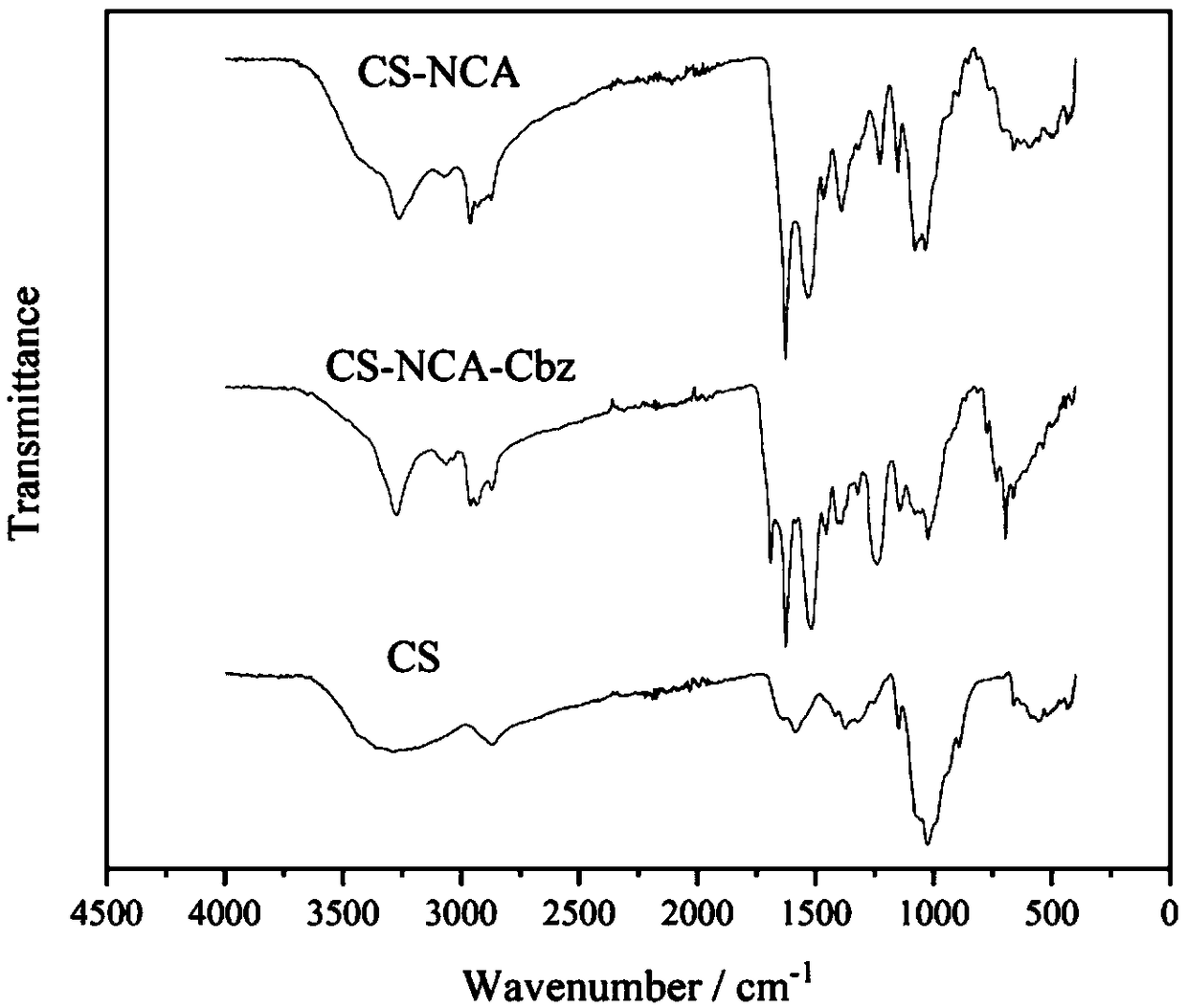
[0019] 1) Synthesis of amino acid derivatives: two amino acid derivatives, lysine-N-carboxylic anhydride (Lys NCA) and valine-N-carboxylic anhydride (ValNCA), were synthesized from benzyloxycarbonyl lysine and valine and Triphosgene reaction to get. The preparation process of the two amino acid derivatives is similar, and only lysine-N-carboxylic acid anhydride (Lys NCA) is used as an example to illustrate the synthesis process. Under nitrogen protection, a certain amount of benzyloxycarbonyllysine was dissolved in an appropriate amount of anhydrous tetrahydrofuran, accompanied by magnetic stirring. The feed ratio of benzyloxycarbonyllysine to triphosgene is 3:1, and triphosgene is added dropwise into the reaction vessel using a syringe. The mixture was warmed up to 50°C and reacted for 3 hours. After the system was clarified, it was cooled to room temperature, and the precipitate was washed three times with a large amount of n-hexane, and the precipitated product was obtain...
Embodiment 2
[0022] Get the chitosan-g-antibacterial peptide polymer solid that embodiment 1 obtains, be configured into the neutral aqueous solution of different concentration gradient, be respectively 4096 μ g / mL, 2048 μ g / mL, 1024 μ g / mL, 512 μ g / mL, 256 μ g / mL, 128 μg / mL, 64 μg / mL, 32 μg / mL, 16 μg / mL, 8 μg / mL, 4 μg / mL, 2 μg / mL, 0 μg / mL. Under the shaking incubation condition at 37°C, Escherichia coli reached the logarithmic growth phase of the bacteria in the LB culture medium. Diluted in LB culture medium to a bacterial concentration of 10 4 -10 5 CFU mL -1 . Mix 100 μL E. coli culture solution with 400 μL LB culture solution and 500 μL chitosan-g-antimicrobial peptide polymer aqueous solution, and add to the centrifuge tube. After incubation at 37°C for 24 hours, the medium was clear and transparent, and the concentration without obvious bacterial growth was determined as the minimum inhibitory concentration (MIC). The experimental results showed that the MIC value was 512 μg / mL....
Embodiment 3
[0024] Chitosan was prepared in the same way as in Example 1 for chitosan-g-antibacterial peptide polymers, and was configured into neutral aqueous solutions with different concentration gradients, which were 8192 μg / mL, 4096 μg / mL, 2048 μg / mL, and 1024 μg respectively. / mL, 512μg / mL, 256μg / mL, 128μg / mL, 64μg / mL, 32μg / mL, 16μg / mL, 8μg / mL, 4μg / mL, 2μg / mL, 0μg / mL. Under the shaking incubation condition at 37°C, Escherichia coli reached the logarithmic growth phase of the bacteria in the LB culture medium. Diluted in LB culture medium to a bacterial concentration of 10 4 -10 5 CFU mL -1 . Mix 100 μL E. coli culture solution with 400 μL LB culture solution and 500 μL chitosan aqueous solution, and add to the centrifuge tube. After incubation at 37°C for 24 hours, the medium was clear and transparent, and the concentration without obvious bacterial growth was determined as the minimum inhibitory concentration (MIC). The results showed that the MIC value was 2048 μg / mL. Dilute ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com