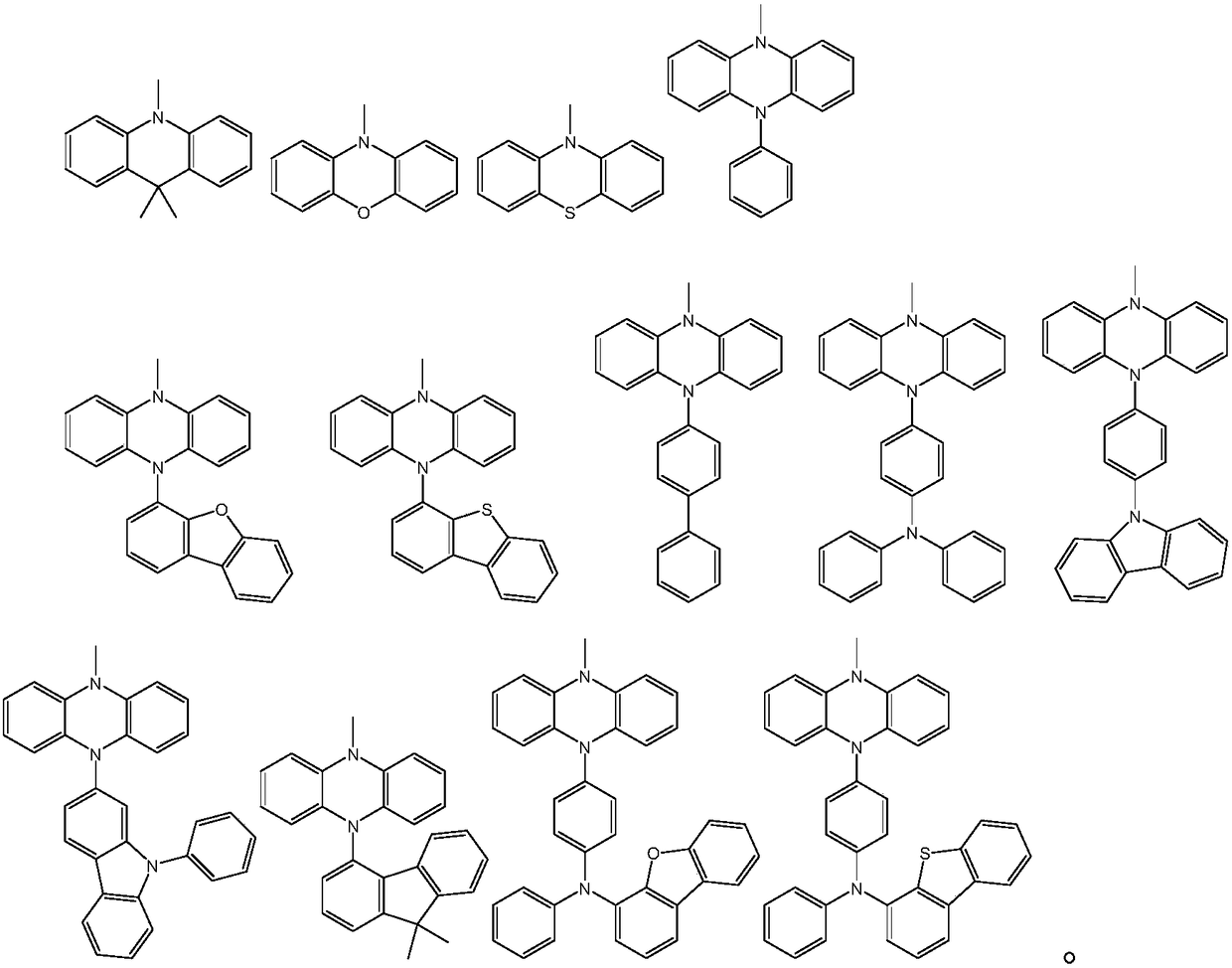
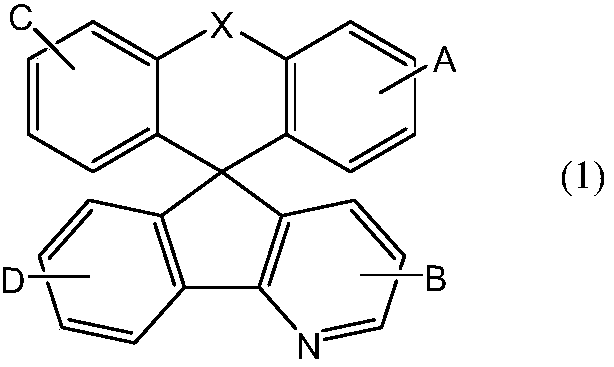
Aza-spirofluorene anthracene heterocyclic compound and application thereof in organic electroluminescence elements
A heterocyclic compound, azafluorene technology, applied in the field of organic electroluminescent functional materials, to achieve the effect of improving triplet energy, low voltage, and improving luminous stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
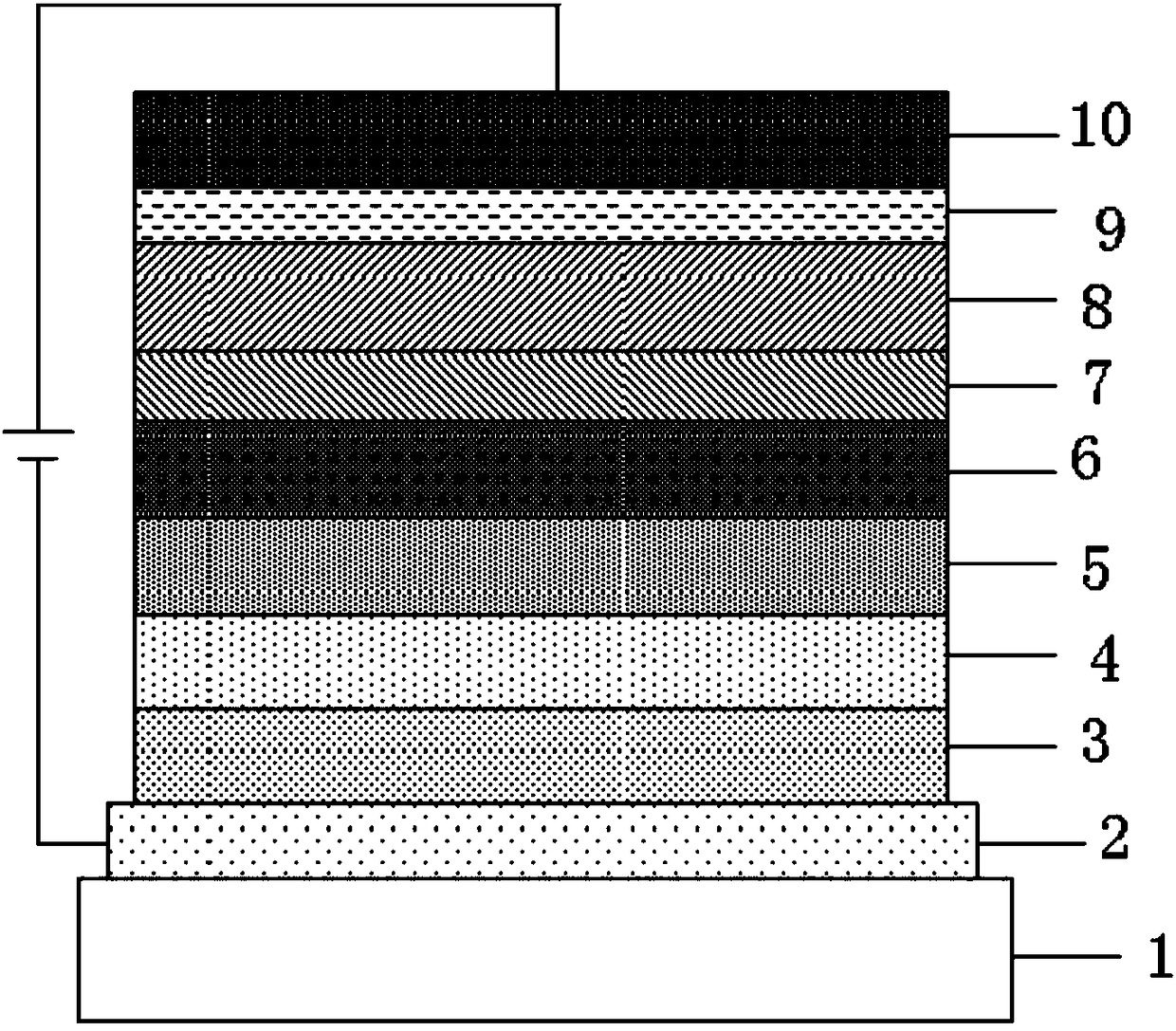
Image
Examples
Embodiment 1
[0086]
[0087] Add 30g of compound 1, 12.6g of phenol, 300ml of dimethylformamide (DMF), 10ml of pyridine into a 500ml three-necked flask, and blow nitrogen for 15min, stir to dissolve the raw materials completely, add 26.4g of potassium carbonate, and 1.2g of cuprous iodide , stirred for 5 minutes, turned on and heated to 100°C, stirred and reacted for 8 hours, then took samples for monitoring, and cooled to room temperature after the raw materials were completely reacted. Add 2M hydrochloric acid solution to adjust the pH to acidic, a large amount of white solid precipitates, extract 3×500ml with ethyl acetate, combine the organic phases, wash with water until neutral, dry with anhydrous magnesium sulfate and spin dry the solvent, the obtained white solid is 32.8g dried in the air, The yield was 88.2%, and it was directly used in the next reaction without further treatment.
[0088] 1 H NMR (400MHz, CDC13) δ7.98 (d, J = 8.4, 1H), 7.36 (dd, J = 8.4, 1H), 7.30 (s, 1H), 7....
Embodiment 2
[0099]
[0100] Add 20g of compound 5 and 150ml of THF to a 500ml three-necked flask successively, blow in nitrogen gas, and cool to 0°C. 125ml of 1.0M in THF phenylmagnesium bromide solution was slowly added dropwise. After raising the temperature to room temperature and continuing the reaction for 2 h, sampling was carried out for detection. When the reaction of the raw materials was complete, the stirring was stopped, and aqueous ammonium chloride solution was added to quench the reaction. Static liquid separation, the aqueous phase was extracted once with dichloromethane, the organic phases were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, and then purified by column to obtain 17.6 g of a yellow solid with a yield of 63.7%.
[0101] 1 H NMR (400MHz, CDC13) δ 9.13 (d, J = 8.4, 1H), 8.58 (d, J = 8.4, 1H), 8.04 (t, J = 8.4, 1H), 7.81 (d, J = 7.6, 2H), 7.54(t, J=7.6, 1H), 7.45(t, J=7.6, 2H);
[0102]
[0103] Add 16g compound 6, 20.3g...
Embodiment 3
[0112]
[0113] Add 8g compound 8, 8.2g diphenylamine, 6.7g potassium carbonate, 0.2g 1,10-phenanthroline, 100ml toluene in a 250ml three-necked flask, feed nitrogen, then add 0.2g cuprous bromide, and the reaction system is heated to The reaction was refluxed and stirred for 8 hours. After the complete reaction of the raw materials was monitored by TLC, it was cooled to room temperature, washed with water to neutrality, the organic phase was dried with anhydrous sodium sulfate for 2 hours and then filtered. After the concentrated solvent was passed through a silica gel column, toluene was recrystallized to obtain the target compound 3 as a white solid 9.5 g, yield 87.7%.
[0114] 1 H NMR (400MHz, CDC13) δ8.54 (d, J = 8.4, 1H), 7.87 (d, J = 7.6, 1H), 7.41 (d, J = 7.6, 1H), 7.14-7.16 (m, 3H) , 7.01(t, J=7.6, 8H), 6.89(t, J=8.4, 1H), 6.77(m, 2H), 6.62(t, J=7.6, 4H), 6.46(t, J=7.6, 8H ), 6.06(dd, J=7.6, 2H), 6.00(s, J=7.6, 2H);
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com