Preparation method of alkyl imidazoline organosilicon quaternary ammonium salt
A technology of organosilicon quaternary ammonium salt and alkyl imidazoline, which is applied in the field of organic synthesis, can solve the problems of non-compliance with green development, ecological development, difficult biodegradation, high price, etc., and achieve good high and low temperature resistance, improve bulkiness, The effect of reducing static electricity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
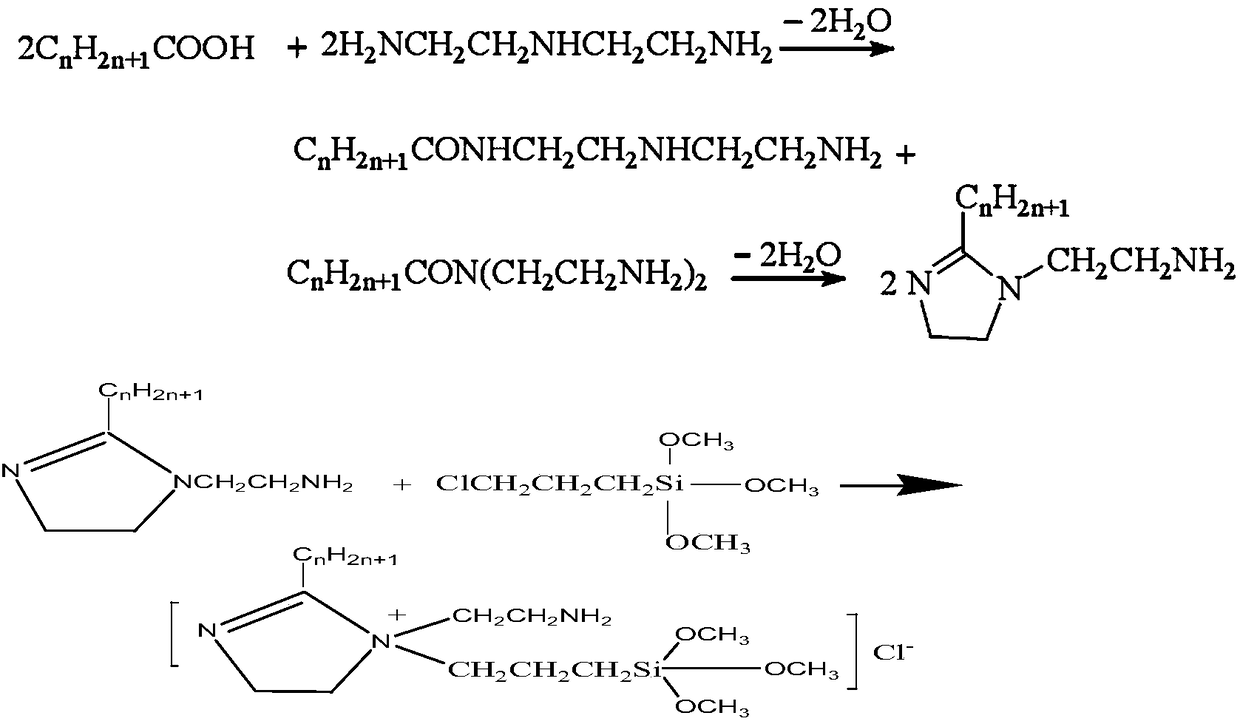
[0026] A preparation method of alkyl imidazoline organosilicon quaternary ammonium salt, the method steps are as follows:
[0027] S1: amidation reaction: weigh long-chain alkyl fatty acid, diethylenetriamine and xylene, heat and react for 6h, pass nitrogen, and the reaction temperature is 155°C; the molar ratio of long-chain alkyl fatty acid to diethylenetriamine is 1 : 1.1, the addition amount of xylene is 70% of the total mass of long-chain alkyl fatty acid and diethylenetriamine, and the long-chain alkyl fatty acid is lauric acid.
[0028] S2: cyclization reaction: continue the reaction at 220°C for 4h, finish the reaction, cool to 120°C, evaporate the residual xylene to obtain a pale yellow liquid, recrystallize three times with acetone to obtain a white solid, and dry to obtain a white powder, obtain Alkyl imidazoline, the yield of long-chain alkyl imidazoline is 86.7%;
[0029] S3: quaternization reaction: add the alkyl imidazoline, halogenated organosilane and catalys...
Embodiment 2
[0032] A preparation method of alkyl imidazoline organosilicon quaternary ammonium salt, the method steps are as follows:
[0033] S1: Amidation reaction: weigh long-chain alkyl fatty acid, diethylenetriamine and xylene, heat and react for 7h, pass nitrogen, and the reaction temperature is 160°C; the molar ratio of long-chain alkyl fatty acid to diethylenetriamine is 1 : 1.2, the addition amount of xylene is 75% of the total mass of long-chain alkyl fatty acid and diethylenetriamine, and the long-chain alkyl fatty acid is myristic acid.
[0034] S2: cyclization reaction: the temperature was increased to 230°C and the reaction was continued for 5h, the reaction was completed, cooled to 120°C, and the residual xylene was evaporated to obtain a pale yellow liquid, which was recrystallized with acetone for 3 times to obtain a white solid, and dried to obtain a white powder, obtained Alkyl imidazoline, the yield of long-chain alkyl imidazoline is 85.3%;
[0035] S3: quaternization...
Embodiment 3
[0038] A preparation method of alkyl imidazoline organosilicon quaternary ammonium salt, the method steps are as follows:
[0039] S1: Amidation reaction: weigh long-chain alkyl fatty acid, diethylenetriamine and xylene, heat and react for 7.5h, pass nitrogen, and the reaction temperature is 165°C; the molar ratio of long-chain alkyl fatty acid to diethylenetriamine is 1:1.15, the addition amount of xylene is 80% of the total mass of long-chain alkyl fatty acid and diethylenetriamine, and the long-chain alkyl fatty acid is palmitic acid.
[0040]S2: cyclization reaction: heat up to 235°C and continue the reaction for 6h, finish the reaction, cool to 120°C, evaporate the residual xylene to obtain a pale yellow liquid, recrystallize with acetone for 3 times to obtain a white solid, and dry to obtain a white powder, obtain Alkyl imidazoline, the yield of long-chain alkyl imidazoline is 87.2%;
[0041] S3: Quaternization reaction: add the alkyl imidazoline, halogenated organosila...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com