L-proline-4-hydroxylase and genetic engineering bacterium thereof, construction method and application thereof
A technology of genetically engineered bacteria and proline, applied in the field of genetic engineering, can solve problems such as low enzyme activity, unused industrial application, and limited strain application, and achieve good genetic stability, strong fermentation acid production ability, and good industrial production. The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The present invention provides a novel high-efficiency L-proline-4-hydroxylase, the amino acid sequence of which is shown in SEQ ID No.2 and derived from Micromonospora sp. CNB394. The screening work of this enzyme is as follows: According to the amino acid sequence (GenBank: BAA20094.1) of L-proline-4-hydroxylase (GenBank: BAA20094.1) that is derived from the L-proline-4-hydroxylase reported at present, compares by BLAST in NCBI database, selects Some genes derived from other microorganisms with a certain degree of similarity in amino acid sequence were identified. Then, these genes were codon-optimized according to the codon preference of Escherichia coli, and the codon-optimized genes were connected to the pTrc99a expression vector through gene synthesis. The constructed vector was introduced into E.coli W3110 which had already accumulated a certain amount of L-proline by electrotransformation. Then the above-mentioned constructed strains are subjected to shake flas...
Embodiment 2
[0043] Construction of strain E.coli HYP:
[0044] 1 Methods of gene editing
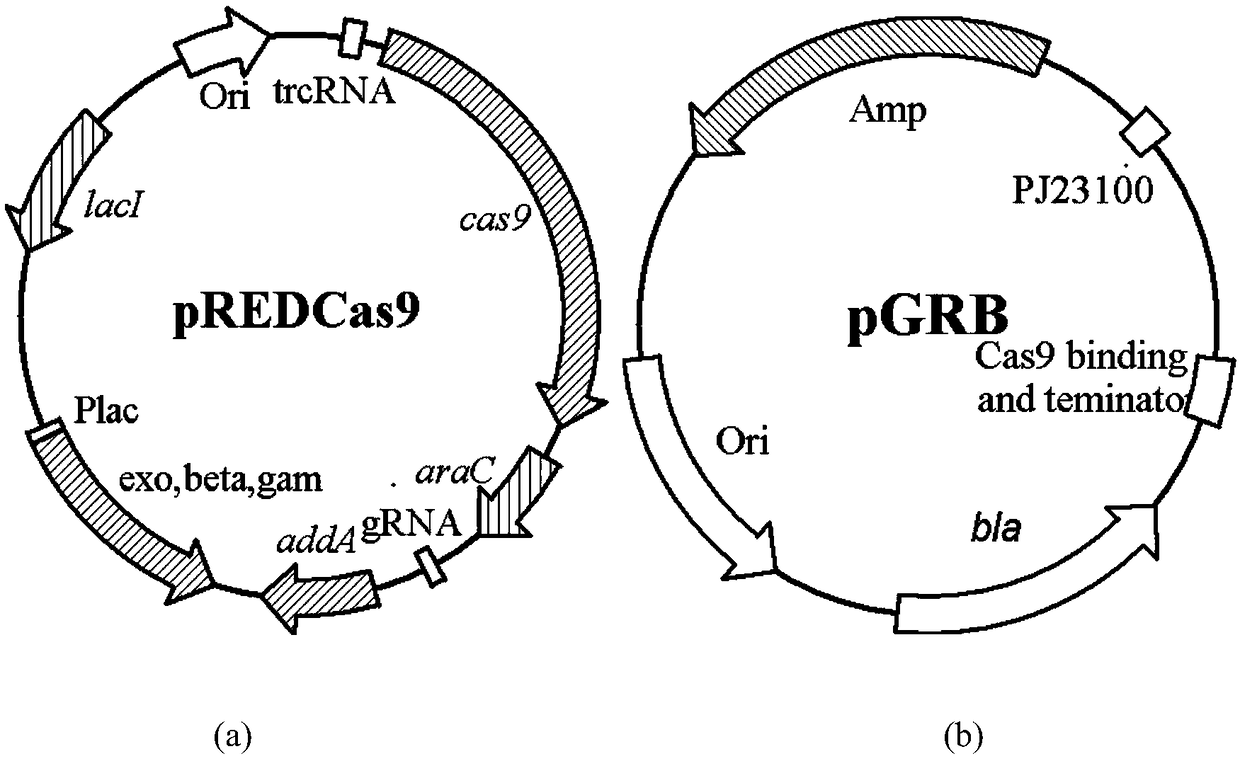
[0045] The gene editing method used in the present invention is carried out with reference to the literature (Li Y, Lin Z, Huang C, et al. Metabolic engineering of Escherichia coli using CRISPR–Cas9 edited genome editing [J]. Metabolic engineering, 2015, 31:13-21.) , the two plasmid maps used in this method are shown in the attached figure 1 . Among them, pREDCas9 carries gRNA expression plasmid pGRB elimination system, bacteriophage λ Red recombination system and Cas9 protein expression system, spectinomycin resistance (working concentration: 100mg / L), cultured at 32°C; pGRB uses pUC18 as the backbone, including the promoter J23100, gRNA-Cas9 binding region sequence and terminator sequence, ampicillin resistance (working concentration: 100mg / L), cultured at 37°C.
[0046] The concrete steps of this method are as follows:
[0047] 1.1. pGRB plasmid construction
[0048] The purpose of construct...
Embodiment 3
[0110] Shake flask fermentation experiment of strain E.coli HYP:
[0111] Slant culture: Streak inoculation of -80°C preserved strains on the activated slant, culture at 37°C for 12 hours, and passage once;
[0112] Shake flask seed culture: Scrape a ring of slant seeds with an inoculation loop and inoculate in a 500mL Erlenmeyer flask containing 30mL of seed medium, seal with nine layers of gauze, and incubate at 37°C and 200rpm for 7-10h;
[0113] Shake flask fermentation culture: Inoculate 10-15% inoculum into a 500mL Erlenmeyer flask (final volume is 30mL), seal with nine layers of gauze, 37°C, 200r / min shaking culture, maintain pH by adding ammonia water during fermentation At 7.0-7.2; add 60% (m / v) glucose solution to maintain fermentation; fermentation cycle 24-30h;
[0114] The composition of the slant medium is: glucose 1-5g / L, peptone 5-10g / L, beef extract 5-10g / L, yeast powder 1-5g / L, NaCl 1-2.5g / L, agar 15-20g / L , the rest is water, pH 7.0-7.2;
[0115] The comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com