Oral attenuated lyophilized vaccine for swine fever and preparation method thereof
A swine fever attenuating virus and swine fever mouth technology, applied in biochemical equipment and methods, vaccines, freeze-dried transportation, etc., can solve problems such as unfavorable growth of pigs, and achieve high antibody titers, convenient administration, and good immune effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
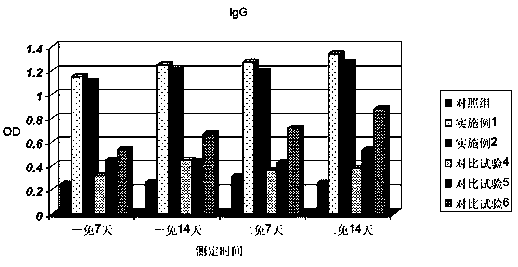
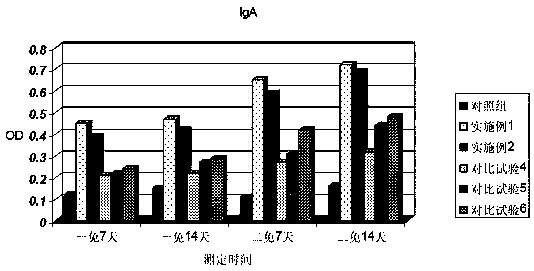
Image
Examples
Embodiment 1
[0034] Embodiment 1: a kind of oral attenuated lyophilized vaccine for classical swine fever, comprising attenuated classical swine fever virus, lyoprotectant and vaccine diluent, is characterized in that, comprises mucosa immune adjuvant and immunopotentiator in the described vaccine diluent, so The mucosal immune adjuvant includes propolis extract, levamisole and carbomer, and the immune enhancer includes astragalus polysaccharide and polygonatum polysaccharide.
[0035] The lyoprotectant includes 3% bovine serum albumin BSA, 2% mannitol, 2% glycine, 5% lactose and 88% deionized water in mass percent.
[0036] Each liter of the vaccine diluent contains: 130mM sodium chloride, 3.0mM potassium chloride, 10mM disodium hydrogen phosphate, 2mM sodium dihydrogen phosphate, 1.2g carbomer, 2.4g levamisole, 4g propolis extract, 5g Astragalus polysaccharide, Polygonatum Polysaccharide 2g.
[0037] The preparation method of the vaccine diluent is: dissolving sodium chloride, potassium...
Embodiment 2
[0047] Embodiment 2: a kind of oral attenuated lyophilized vaccine for classical swine fever, comprising attenuated classical swine fever virus, lyoprotectant and vaccine diluent, is characterized in that, comprises mucosa immune adjuvant and immunopotentiator in the described vaccine diluent, so The mucosal immune adjuvant includes propolis extract, levamisole and carbomer, and the immune enhancer includes astragalus polysaccharide and polygonatum polysaccharide.
[0048] The lyoprotectant includes 3% bovine serum albumin BSA, 2% mannitol, 2% glycine, 6% lactose and 87% deionized water in mass percent.
[0049] Each liter of the vaccine diluent contains: 130mM sodium chloride, 3.0mM potassium chloride, 10mM disodium hydrogen phosphate, 2mM sodium dihydrogen phosphate, 1.5g of carbomer, 3.0g of levamisole, 2g of propolis extract , 3g of Astragalus Polysaccharide, 1.5g of Polygonatum polysaccharide.
[0050] The preparation method of the vaccine diluent is: dissolving sodium c...
Embodiment 3
[0060] Embodiment 3: a kind of oral attenuated lyophilized vaccine for classical swine fever, comprising attenuated classical swine fever virus, lyoprotectant and vaccine diluent, is characterized in that, comprises mucosa immune adjuvant and immunopotentiator in the described vaccine diluent, so The mucosal immune adjuvant includes propolis extract, levamisole and carbomer, and the immune enhancer includes astragalus polysaccharide and polygonatum polysaccharide.
[0061] The lyoprotectant includes 3% bovine serum albumin BSA, 2% mannitol, 2% glycine, 5% lactose and 88% deionized water in mass percent.
[0062] Each liter of the vaccine diluent contains: 130mM sodium chloride, 3.0mM potassium chloride, 10mM disodium hydrogen phosphate, 2mM sodium dihydrogen phosphate, 1.4g carbomer, 2.8g levamisole, 3g propolis extract, 2g Astragalus polysaccharide, Polygonatum Polysaccharide 1g.
[0063] The preparation method of the vaccine diluent is: dissolving sodium chloride, potassium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com