Preparation method of magnetic composite hydrogel for copper ion adsorption
A composite hydrogel, copper ion technology, applied in the fields of adsorption water/sewage treatment, chemical instruments and methods, water pollutants, etc., can solve the problems of weak adsorption performance, poor hydrophilicity, affecting adsorption capacity, etc., and achieve adsorption capacity. The effect of improving, increasing porosity, and increasing adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
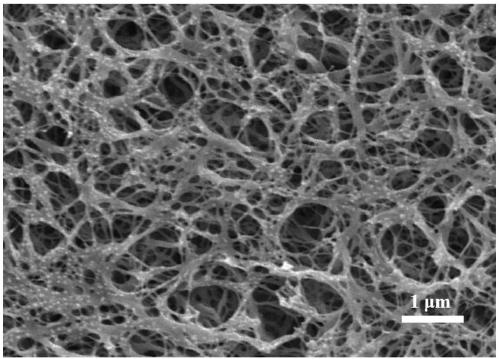
[0030] 1) Preparation of cellulose nanofibers
[0031] Quenching solution preparation: add 0.28g cellulose acetate to 2g tetrahydrofuran and 5g N,N'-dimethylformamide mixed solvent, magnetically stir and dissolve at room temperature to obtain quenching solution;
[0032] Take 5mL of quenching solution and pour it into a petri dish with a diameter of 5cm, put the petri dish in the refrigerator, and quench at -30°C for 2.7h. After the end, quickly take it out and put it into a 500mL ice-water mixture, and change the distilled water every 6h, 4 times. Finally, freeze-drying obtains cellulose acetate nanofibers;
[0033] The cellulose acetate nanofibers were soaked in 0.1mol / L NaOH / ethanol solution for 24h, and then washed with distilled water until the pH value of the washing liquid was 7. Cellulose nanofibers were obtained by freeze-drying.
[0034] 2) Preparation of cellulose nanofiber grafted poly(acrylic acid-co-maleic anhydride) hydrogel.
[0035] Dissolve 1 g of vinyltr...
Embodiment 2
[0042] 1) Preparation of cellulose nanofibers
[0043] Quenching liquid preparation: add 0.20g cellulose acetate to 2g tetrahydrofuran and 5g N,N'-dimethylformamide mixed solvent, magnetically stir and dissolve at room temperature to obtain quenching liquid;
[0044] Take 5mL of quenching solution and pour it into a petri dish with a diameter of 5cm, put the petri dish in the refrigerator, and quench at -15°C for 2.5h. After the end, quickly take it out and put it into a 500mL ice-water mixture, and change the distilled water every 6h, 4 times. Finally, freeze-drying obtains cellulose acetate nanofibers;
[0045] The cellulose acetate nanofibers were soaked in 0.08mol / L NaOH / ethanol solution for 24h, and then washed with distilled water until the pH value of the washing solution was 7. Cellulose nanofibers were obtained by freeze-drying.
[0046] 2) Preparation of cellulose nanofiber grafted poly(acrylic acid-co-maleic anhydride) hydrogel.
[0047] Dissolve 1 g of vinyltri...
Embodiment 3
[0053] 1) Preparation of cellulose nanofibers
[0054] Quenching solution preparation: add 0.20g cellulose acetate to 2g tetrahydrofuran and 4g N,N'-dimethylformamide mixed solvent, magnetically stir and dissolve at room temperature to obtain quenching solution;
[0055] Take 5mL of quenching solution and pour it into a petri dish with a diameter of 5cm, put the petri dish in the refrigerator, and quench at -20°C for 2.5h. After the end, quickly take it out and put it into a 500mL ice-water mixture, and change the distilled water every 6h, 4 times. Finally, freeze-drying obtains cellulose acetate nanofibers;
[0056] The cellulose acetate nanofibers were soaked in 0.15mol / L NaOH / ethanol solution for 24h, and then washed with distilled water until the pH value of the washing solution was 7. Cellulose nanofibers were obtained by freeze-drying.
[0057] 2) Preparation of cellulose nanofiber grafted poly(acrylic acid-co-maleic anhydride) hydrogel.
[0058] Dissolve 1 g of viny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
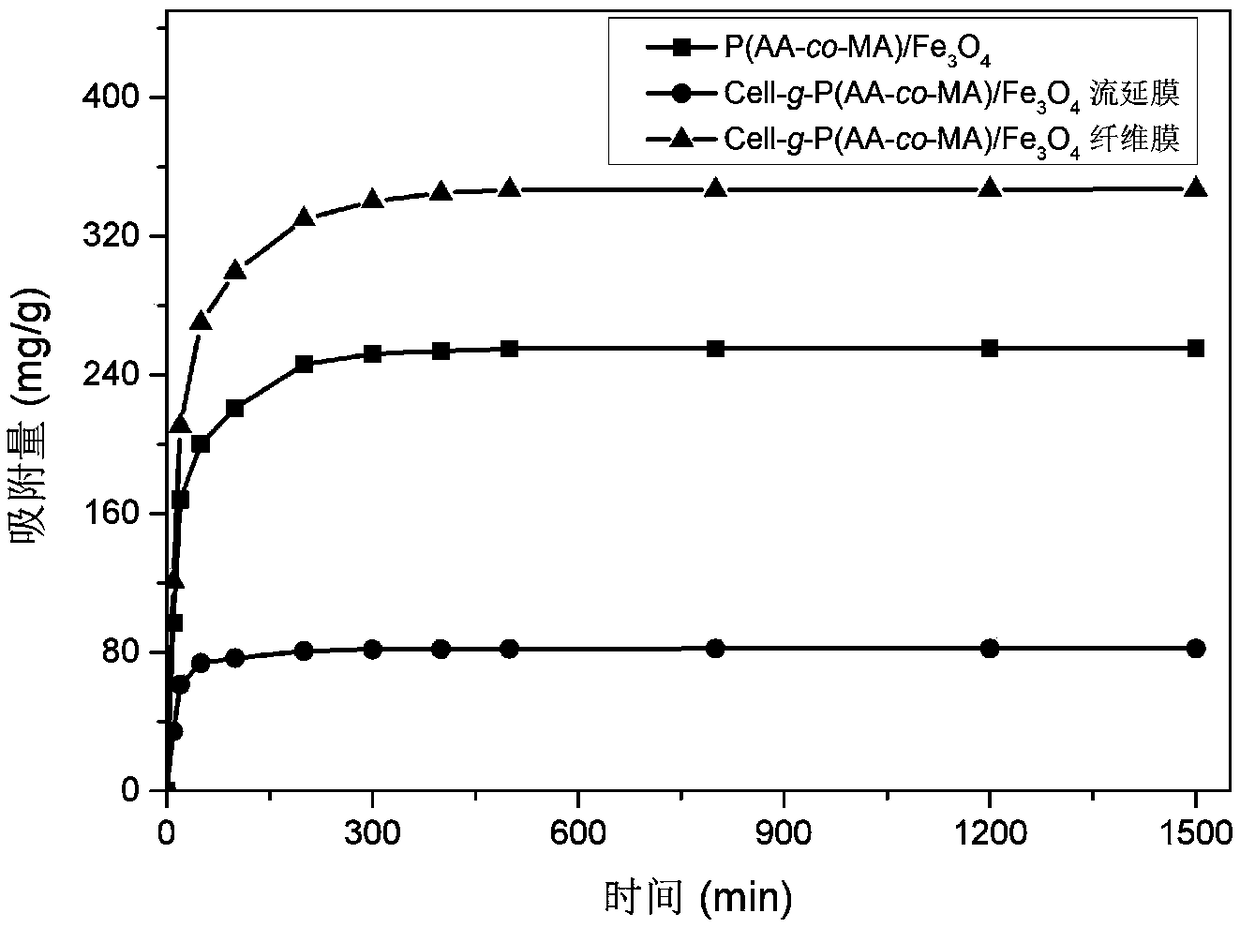
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com