A hydroxide ion exchange membrane for preparing hydrogen for fuel cells and a preparation method thereof
A technology for ion exchange membranes and fuel cells, which is applied in the field of preparation of hydroxide ion exchange membranes for hydrogen used in fuel cells and its preparation. It can solve the problems of difficult control of electrical conductivity and mechanical properties, low tolerance, and complicated preparation. Achieve the effects of improving hydrophilicity and anion permeability, improving tolerance and stability, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Under nitrogen atmosphere, mix 17kg N-vinylimidazole and 13 kg iodomethane, stir at 150r / min for 33min, then add 27 kg styrene, 40 kg acrylonitrile, 0.5 kg benzoyl isobutyl ether, 2 kg of benzoyl peroxide and 0.5 kg of triethanolamine were ultrasonically oscillated at a frequency of 260 kHz for 17 minutes to obtain a homogeneous solution; then the glass mold was immersed in the homogeneous solution. 2 Irradiate under ultraviolet light at a distance of 42cm for 7min. After the reaction is completed, take out the glass mold, wash it with deionized water, soak it in sodium hydroxide solution, and heat it in a water bath at 82°C for 13h; finally take out the glass mold, Wash with deionized water until the washing solution is neutral, remove the glass mold, and obtain a hydroxide ion exchange membrane.
[0031] testing method:
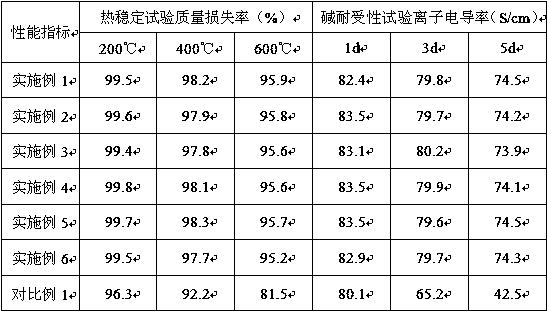
[0032] (1) Thermal stability (mass loss rate): A thermogravimetric analyzer (DMA / SDDTA861e) was used to carry out a thermal stability test, and 10 ...
Embodiment 2
[0036] Under nitrogen atmosphere, mix 15 kg N-vinylimidazole and 12 kg methyl iodide, stir at 130 r / min for 40 min, then add 25 kg styrene, 46 kg acrylonitrile, 0.5 kg benzoyl isobutyl ether, 1 kg of dicumyl peroxide and 0.5kg of 2,4,6-tris(dimethylaminomethyl)phenol were ultrasonically oscillated for 20 minutes under an ultrasonic frequency of 200kHz to obtain a homogeneous solution; then the glass mold was immersed in the homogeneous solution , at an irradiation intensity of 800μW / cm 2 Irradiate under ultraviolet light at a distance of 30cm for 10min. After the reaction is completed, take out the glass mold, wash it with deionized water, soak it in sodium hydroxide solution, and heat it in a water bath at a temperature of 70°C for 14h; finally take out the glass mold, Wash with deionized water until the washing solution is neutral, remove the glass mold, and obtain a hydroxide ion exchange membrane.
[0037] The test method is consistent with Example 1, and the obtained dat...
Embodiment 3
[0039] Under a nitrogen atmosphere, mix 18 kg N-vinylimidazole and 16 kg methyl iodide, stir at 180 r / min for 20 min, then add 30 kg styrene, 32 kg acrylonitrile, 1 kg benzoyl isobutyl ether, 2 kg diethylenetriamine, 1kg triethylenediamine, and ultrasonically vibrate for 15 minutes under ultrasonic frequency of 300kHz to obtain a homogeneous solution; then immerse the glass mold in the homogeneous solution, 2 irradiate with ultraviolet light at a distance of 50cm for 5min, take out the glass mold after the reaction is completed, wash it with deionized water, soak it in sodium hydroxide solution, and heat it in a water bath at a temperature of 90°C for 10h; finally take out the glass mold, Wash with deionized water until the washing solution is neutral, remove the glass mold, and obtain a hydroxide ion exchange membrane.
[0040] The test method is consistent with Example 1, and the obtained data are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com