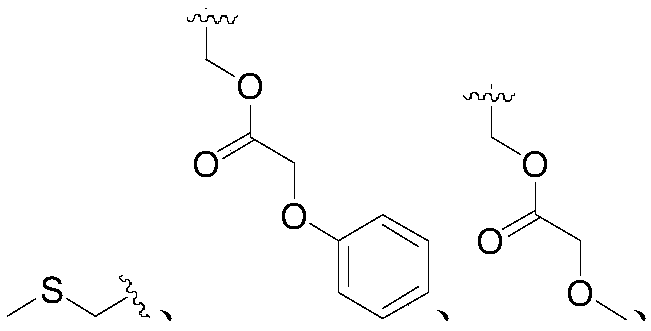
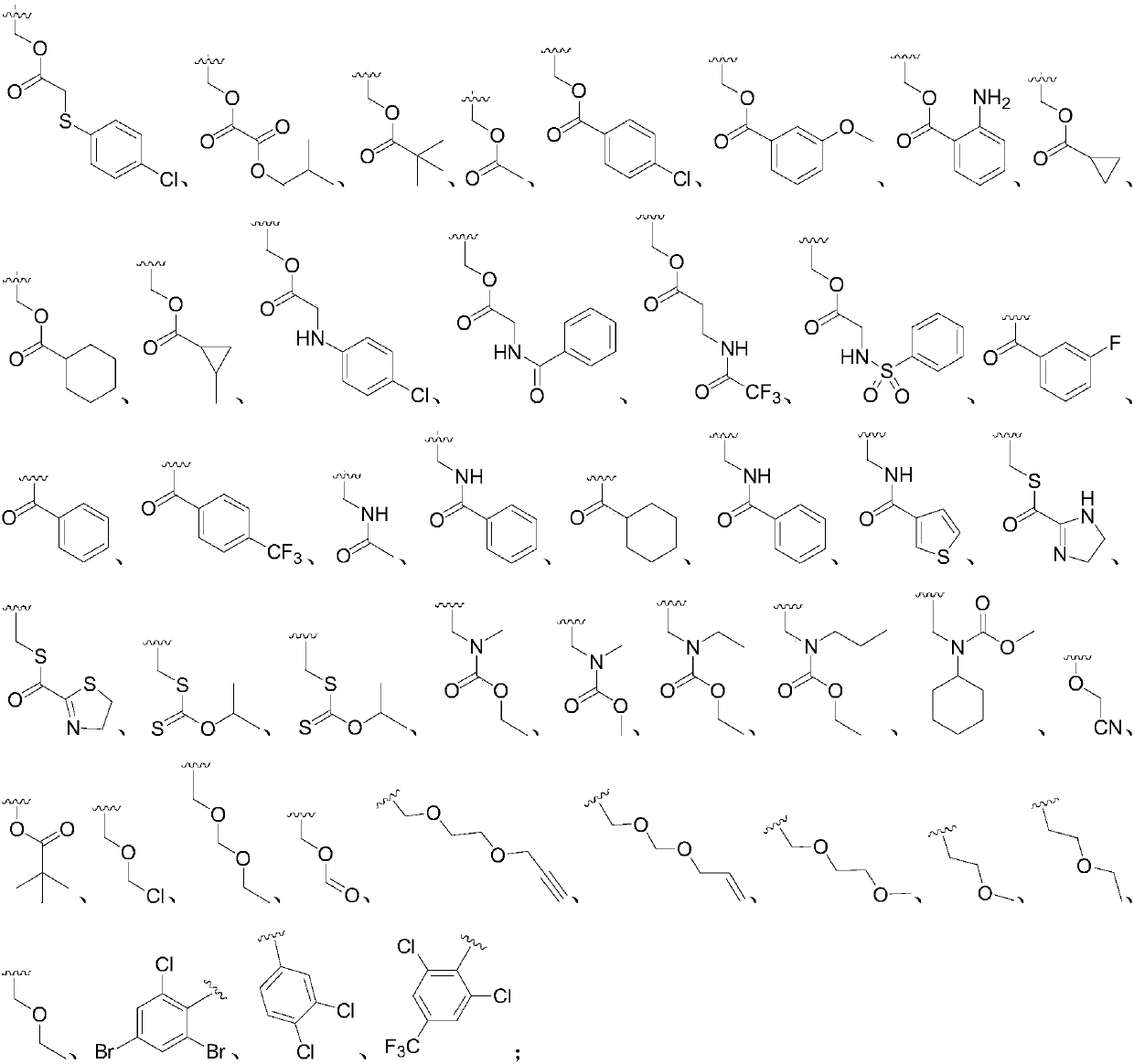
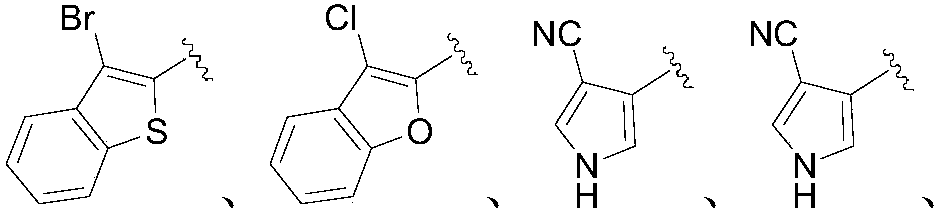
Application of 2-aryl substituted pyrrole compound in medicine for killing oncomelania hupensis
A compound and aryl technology, applied in the field of schistosomiasis control, can solve the problems of slow efficacy of molluscicide, environmental pollution, and less research on killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of compound 5
[0062]
[0063] (1) Synthesis of Triisopropylpyrrole
[0064] Measure 200mLTHF into a 1000mL three-neck flask, cool down to 0°C, slowly add 12.0g (0.500mol) sodium hydride, stir for 10min, add 17.0g (0.253mol) pyrrole dropwise to the suspension, and stir at 0°C for 1.5h , then slowly dropwise added 13.7g (0.259mol) triisopropylchlorosilane, after the addition was completed, stirred for 2h, added ice water, extracted with ethyl acetate, the extract was dried over anhydrous sodium sulfate, suction filtered and concentrated to obtain 51.7 g oil.
[0065] (2) Synthesis of 3-formaldehyde-1H-pyrrole
[0066] Preparation of Vilsmeier reagent: Weigh 3.9 g (0.876 mol) of DMF in a three-neck flask, cool down to 0°C, then weigh 89.6 g (0.584 mol) of phosphorus oxychloride and slowly add it dropwise to DMF. Stir at ℃ for 1.5h.
[0067] Measure an appropriate amount of dichloromethane to completely dissolve the prepared Vilsmeier reagent, cool down t...
Embodiment 2
[0073] Synthesis of compound 7
[0074]
[0075] (1) Synthesis of 2-phenylaziridine:
[0076] Dissolve 10.4g (100mmol) of styrene in 80mL of carbon tetrachloride, dissolve 16g (200mmol) of liquid bromine in 60mL of carbon tetrachloride, keep the styrene solution at 15-20°C in an ice-water bath, and slowly dissolve the liquid bromine solution Add it dropwise to the styrene solution, stir for 2 hours, wash with water, dry, and rotary evaporate under reduced pressure to obtain a white solid (1).
[0077] Dissolve the white solid (1) in 140 mL DMSO, add 9.8 g (150 mmol) sodium azide under nitrogen protection, and stir overnight at room temperature. Then 4 mL of an aqueous solution containing 4 g of sodium hydroxide was added to the mixture, and stirring was continued for 24 h. The reaction product was poured into 400 mL of 2% (wt%) sodium bicarbonate solution, extracted with dichloromethane, the organic layer was repeatedly washed with water, dried and then rotary evaporated ...
Embodiment 3
[0082] Synthesis of Compound 12
[0083]
[0084] Firstly, α-p-chlorophenylglycine was N-formylated with a mixture of formic acid and acetic anhydride, converted to the corresponding N-formyl derivative, and further reacted with 2-chloroacrylonitrile in the presence of acetic anhydride for 1 ,3-dipolar addition reaction to generate the corresponding N-formyl derivatives; further 1,3-dipolar addition reaction with 2-chloroacrylonitrile in the presence of acetic anhydride to generate arylpyrrolenitrile 12 As the main product, the total yield of the two-step reaction is 73.6%, and the product is light yellow solid powder with a melting point of 161-162°C; 1 H NMRδ(in acetone-D 6 ):6.59(t, J=2.7Hz, 1H), 7.05(t, J=2.8Hz, 1H), 7.55(d, J=8.5Hz, 2H, Ar-H), 7.84(d, J=8.3Hz , 2H, Ar-H), 11.30 (bs, 1H, N-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com