Antitumor application of chlorambucil-polydopamine prodrug nanoparticles
A technology of chlorambucil and nanoparticles, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
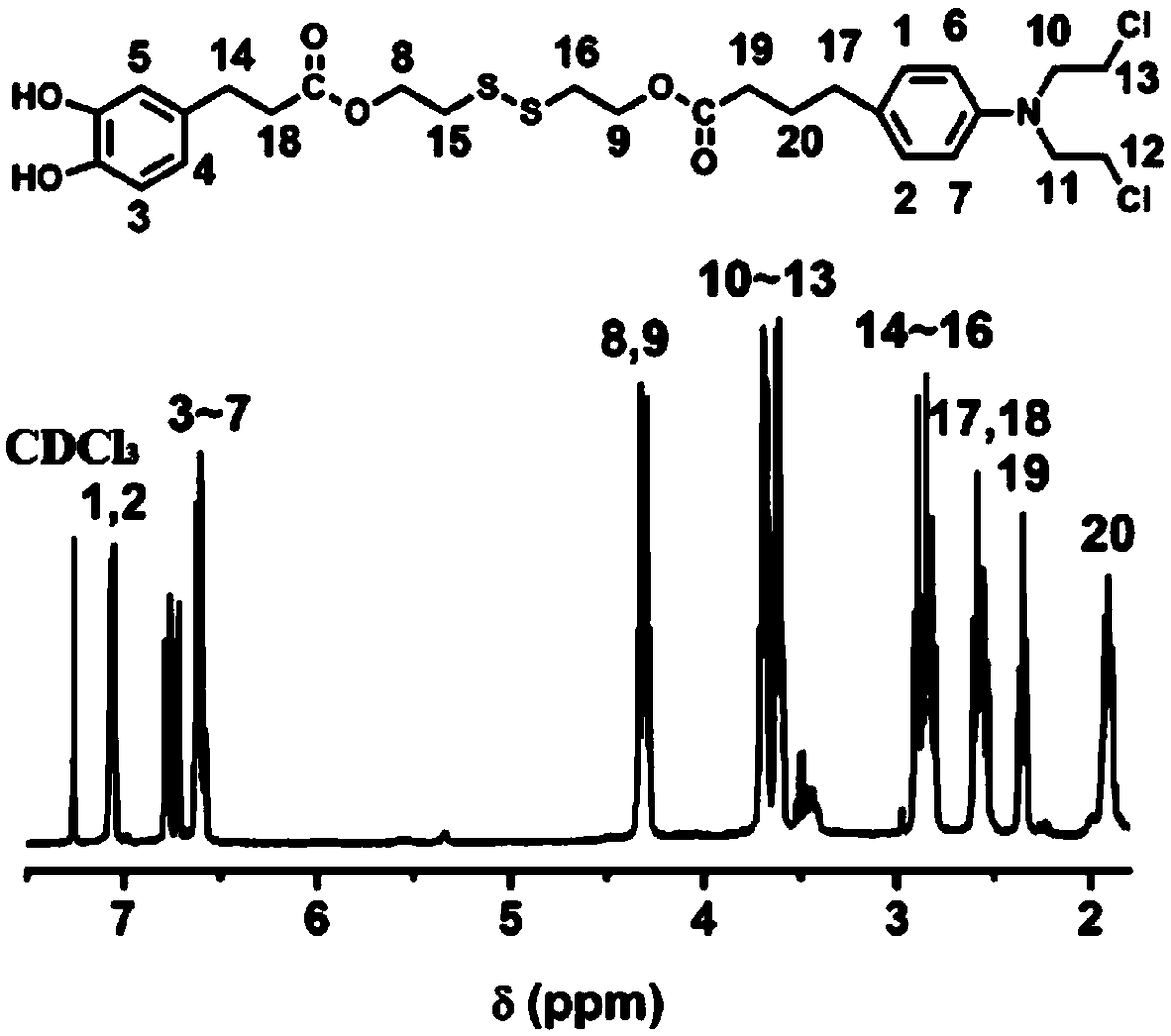
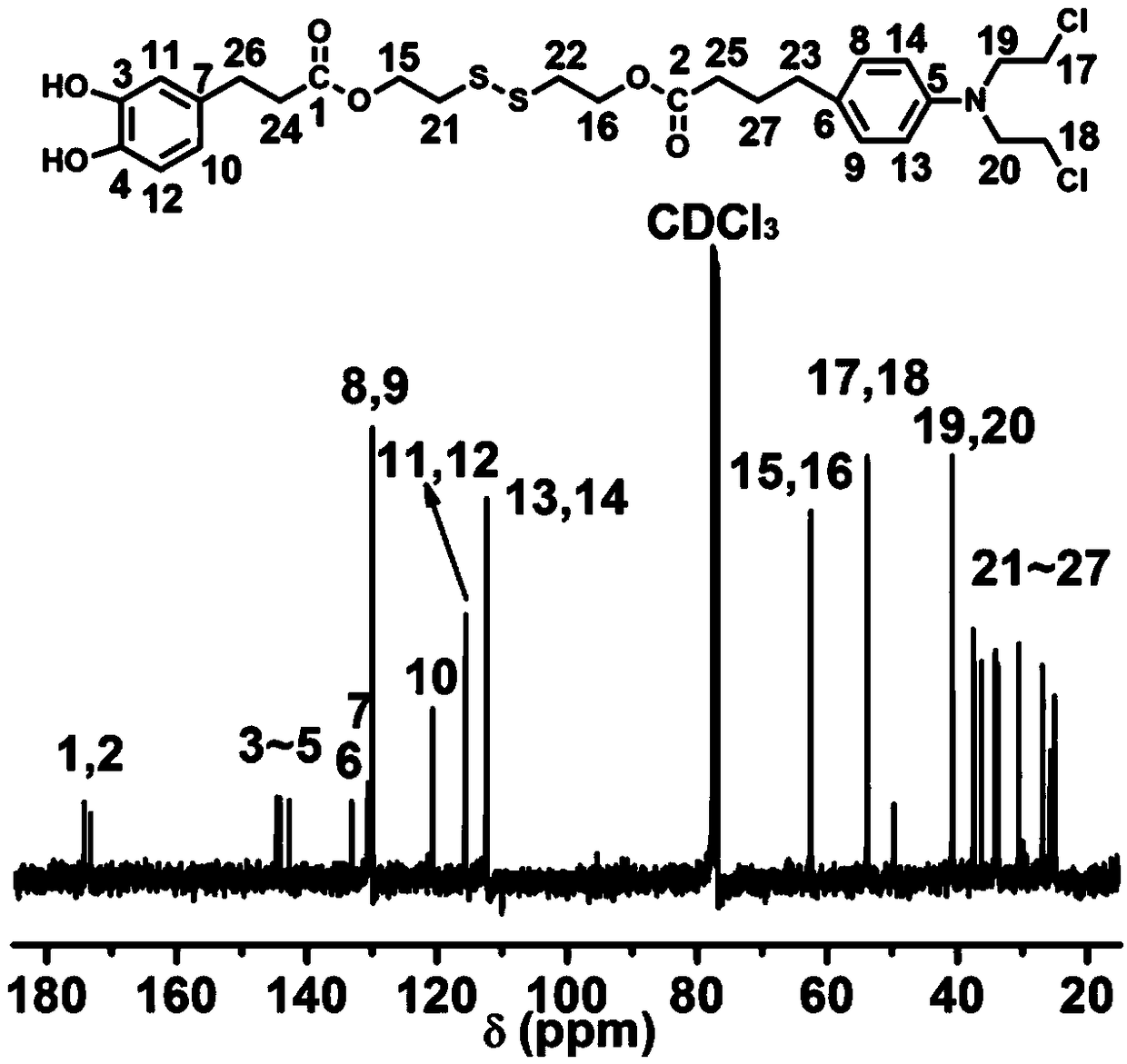
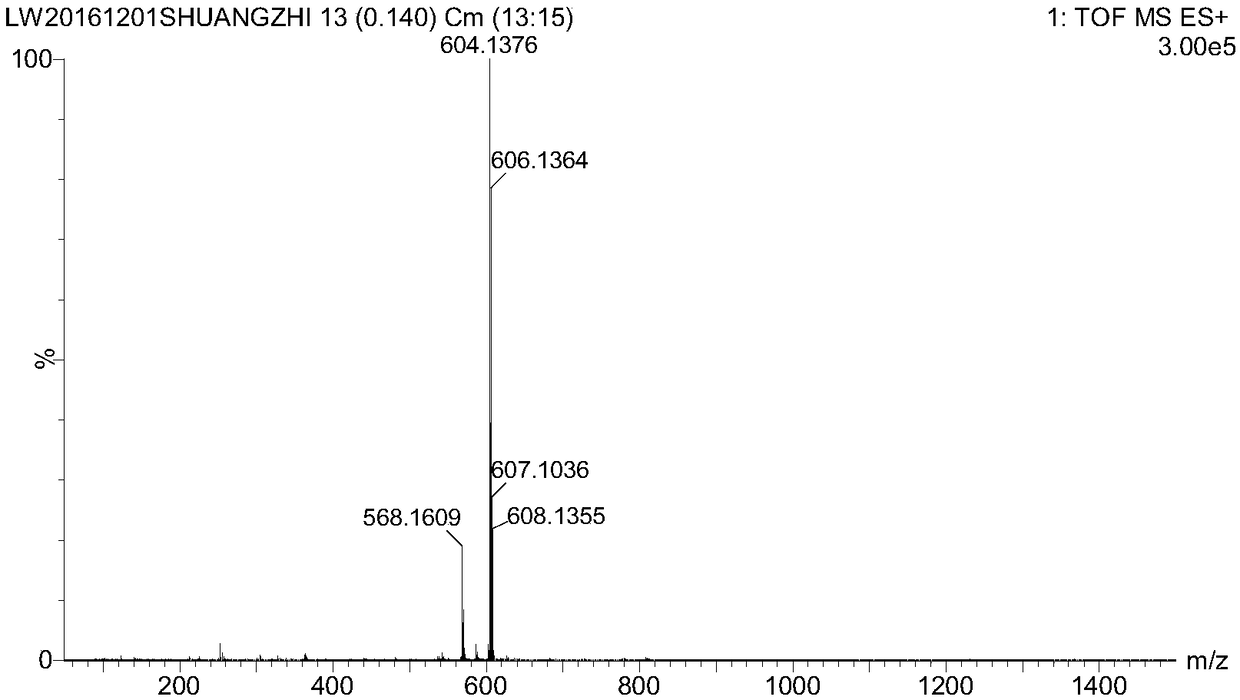
[0074] Example 1 Preparation of chlorambucil-dopamine conjugate molecule
[0075] Step 1, 1000 mg of 3,4-dihydroxyphenylpropionic acid was dissolved in 20 mL of anhydrous acetone, and 383 μL of phosphorus trichloride was slowly added dropwise to the above reaction flask, and the reaction was carried out in an ice-water bath at 0°C for 6 After hours, the water pump was rotovaped. The crude product was dissolved in a mixture of water and ether, extracted with a separating funnel, the organic layer was extracted three times with water, concentrated and dried to obtain a white powder 2,2-dimethyl-1,3-benzodioxolane ene-5-propionic acid. Yield 60%.
[0076] Step 2, 222mg of 2,2-dimethyl-1,3-benzodioxol-5-propionic acid, 247.2mg of dicyclohexylcarbodiimide, 146.4mg of 4-dimethylamino Pyridine was dissolved in 5 mL of anhydrous dichloromethane, and after 4 hours of reaction at room temperature, 180 mg of the dichloromethane solution of 2-hydroxyethyl disulfide was added dropwise t...
Embodiment 2
[0082] Example 2 Preparation of redox-responsive chlorambucil-polydopamine prodrug nanoparticles
[0083] Add 410mg of tris, 20mL of distilled water to a 50mL round bottom flask, stir at 30°C for 30 minutes, dissolve 12.5mg of dopamine hydrochloride and 53.7mg of chlorambucil-dopamine conjugate molecules in 1mL of distilled water, And quickly injected into the above solution, the reaction time is 24 hours, and the nanoparticle solution is black. Distilled water dialysis (dialysis bag molecular weight cut-off 3500) for two days, 1000mL distilled water × 8. Freeze dry for 48 hours. The yield is 10% to 30%.
[0084] The dynamic light scattering pattern of the chlorambucil-polydopamine prodrug nanoparticles that the present embodiment makes is as follows Figure 4 shown.
Embodiment 3
[0085] Example 3 Preparation of redox-responsive chlorambucil-polydopamine prodrug nanoparticles
[0086] The steps of this example are the same as those in Example 2, except that the masses of dopamine hydrochloride and chlorambucil-dopamine conjugate molecules are 12.5 mg and 17.4 mg, respectively. The yield of this embodiment is 30%~40%.
[0087] The dynamic light scattering pattern of the chlorambucil-polydopamine prodrug nanoparticles that the present embodiment makes is as follows Figure 5 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com