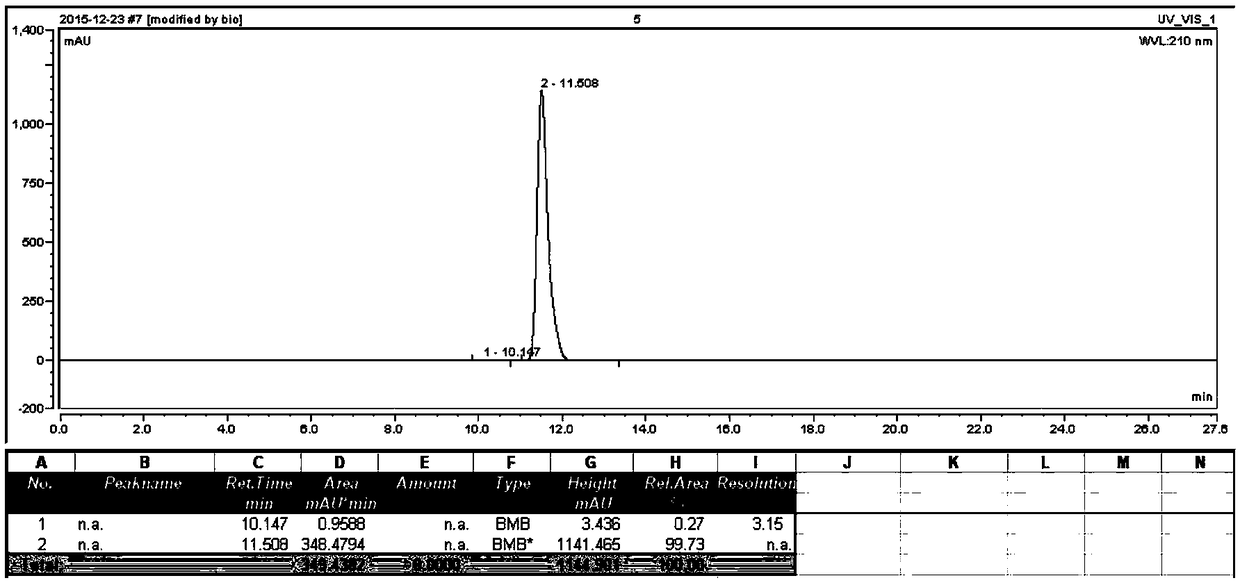
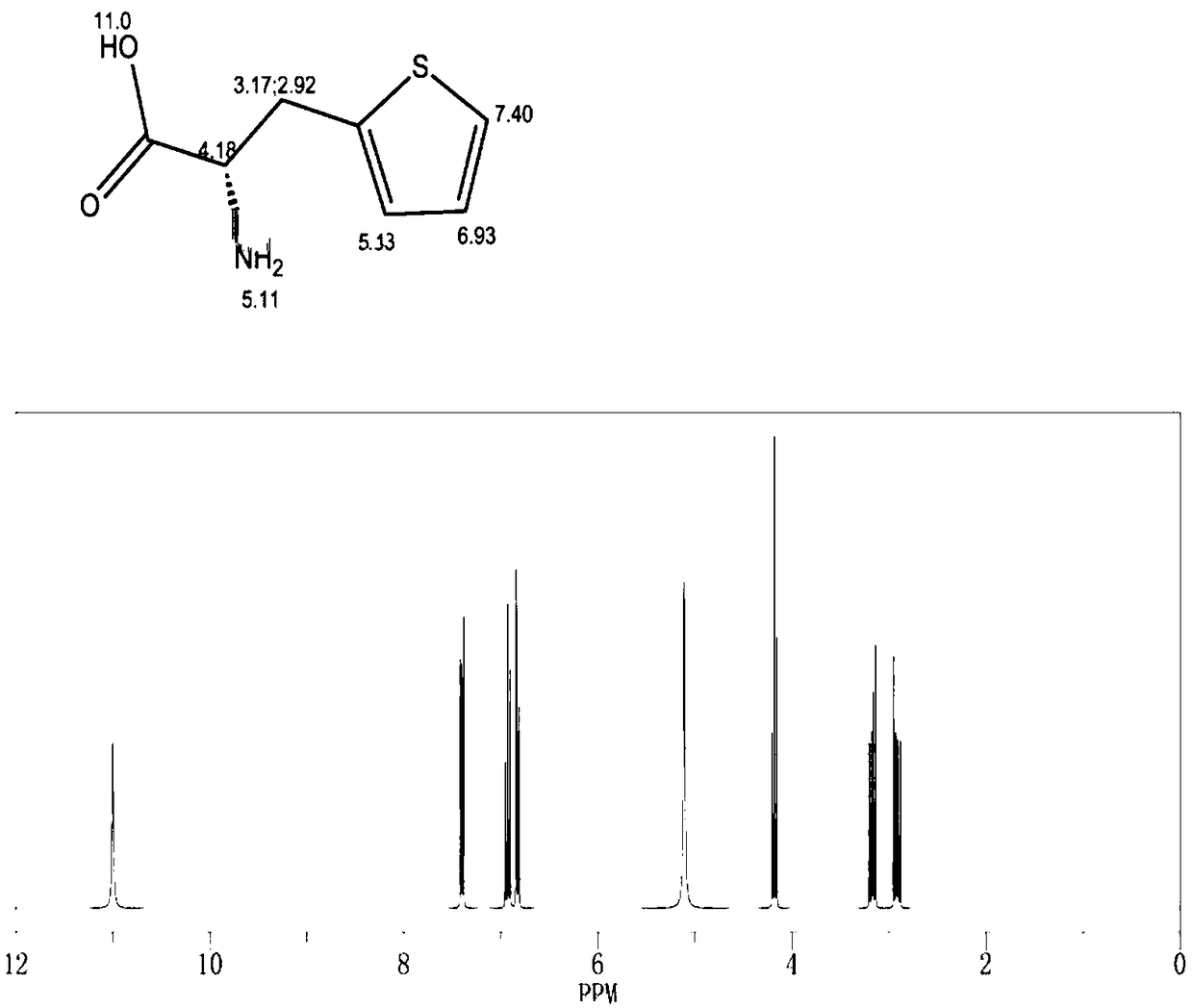
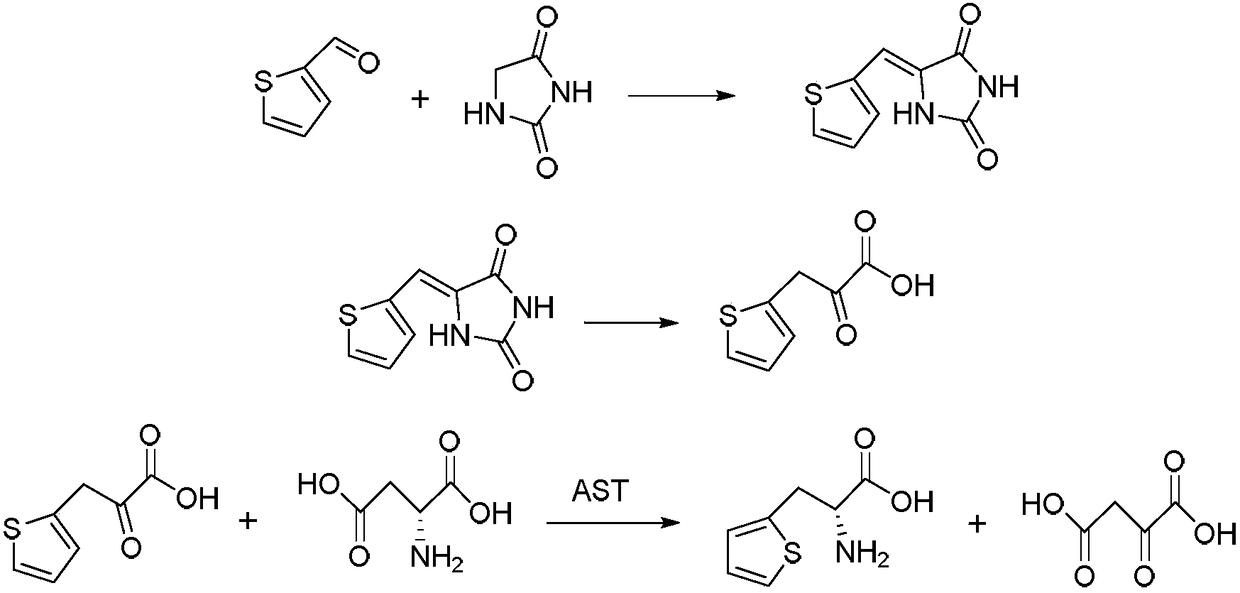
Method for preparing 3-(2-thienyl)-L-alanine
A technology of thiophenepyruvate and alanine, which is applied in the direction of fermentation, can solve the problems of low yield, high cost, and cumbersomeness, and achieve the effects of high yield, low production cost, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of fermentation broth containing L-aspartate aminotransferase:
[0042] Concrete fermentation condition is: the bacterium that produces enzyme is Escherichia coli (Escherichiacolt, ATCC11303), 1.5% glucose, 0.6% peptone, 0.1% beef extract, 2% corn steep liquor, 0.05% sodium chloride, 0.05% magnesium sulfate, 0.05% sulfuric acid Ammonium. Use sodium hydroxide solution to adjust the pH to 7.5-7.7, inoculum amount of seed solution is 5-10% (v / v), culture at 35-37°C for 20-24 hours at 160 rpm, microscopic examination is full, and OD value 0.4-0.6, enzyme activity: 0.5-0.6umol / (ml.min). The enzyme is an intracellular enzyme and does not need to break the wall. After fermentation, the fermentation broth containing bacteria can be used directly, or it can be used after centrifugation.
Embodiment 2
[0044] (1) Add 500ml of water into a 1000ml four-neck flask equipped with a stirring, ventilating and reflux device, then add 100g (1mol) hydantoin and 5g (0.08mol) ethanolamine, stir fully, protect with nitrogen, and keep warm in a water bath for 10 to 15 ℃, 106g (0.95mol) 2-thiophene formaldehyde was added dropwise, and the dropwise addition was completed in 2 hours. After the drop, the temperature was raised to 85°C, and the temperature was kept for 3 hours. It was detected that there was no 2-thiophene formaldehyde in the solution, and the temperature was continued to rise to 95°C. hour, cooled to room temperature, suction filtered, washed with a small amount of water, and dried to obtain 175.2 g of 2-thiophene hydantoin (yield 95.5%, purity 99.4%);
[0045] (2) Add 500ml of water to a 1000ml four-necked flask with stirring, ventilating and reflux devices, then add 50g (0.26mol) 2-thiophene hydantoin and 24g (0.6mol) sodium hydroxide, fully stir, and Under protection, heat...
Embodiment 3
[0049] (1) Add 500ml of water into a 1000ml four-neck flask with a stirring, ventilating and reflux device, then add 100g (1mol) hydantoin and 5.5g (0.073mol) n-propanolamine, stir well, pass nitrogen protection, and water bath Keep warm at 10-15°C, add 106g (0.95mol) 2-thiophene carboxaldehyde dropwise, and finish the dropwise addition in 2 hours, then raise the temperature to 85°C, keep warm for 3 hours, check that there is no 2-thiophene carboxaldehyde in the solution, continue to heat up to 95 ℃, keep warm for 1 hour, cool down to room temperature, filter with suction, wash with a small amount of water, and dry to obtain 171.2 g of 2-thiophene hydantoin (yield 92.7%, purity 99.2%);
[0050] (2) Add 500ml of water to a 1000ml four-necked flask with a stirring, ventilating and reflux device, then add 50g (0.26mol) 2-thiophene hydantoin and 30g (0.75mol) sodium hydroxide, fully stir, and Under protection, the temperature was raised to 90°C for heat preservation and hydrolysis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com