Biodegradable ureteral stents, methods and uses thereof
A biodegradable resin and polymer technology, applied in the field of biodegradable ureteral stents, can solve problems such as poor drug delivery and limited effect of double-J ureteral stents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
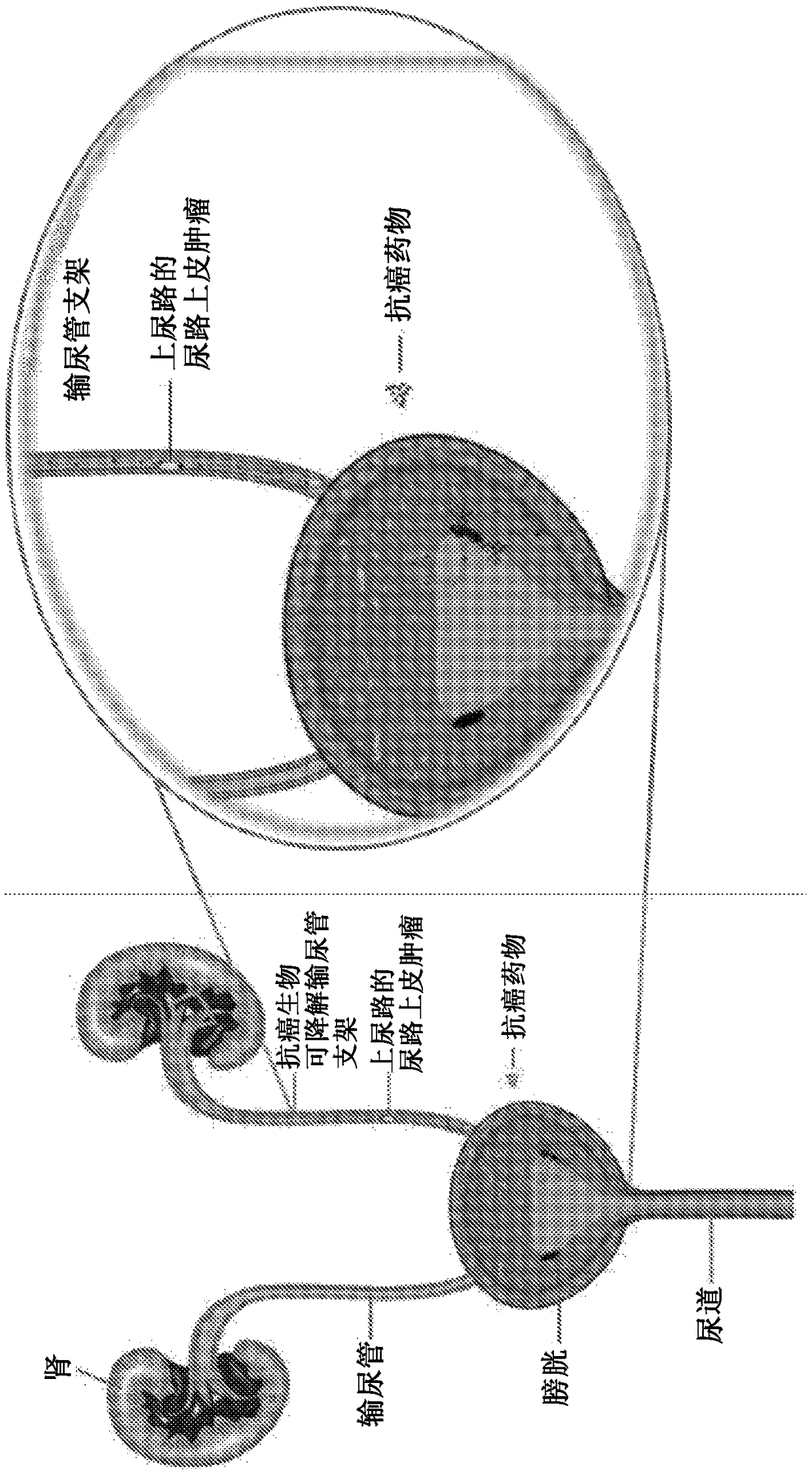
[0050] The present disclosure relates to biodegradable ureteral stents comprising anti-cancer drugs, and compositions useful in medicaments for ensuring the patency of the passage, i.e., mammalian ureters, such as ureters obstructed by urolithiasis, neoplasia, or surgical procedures .
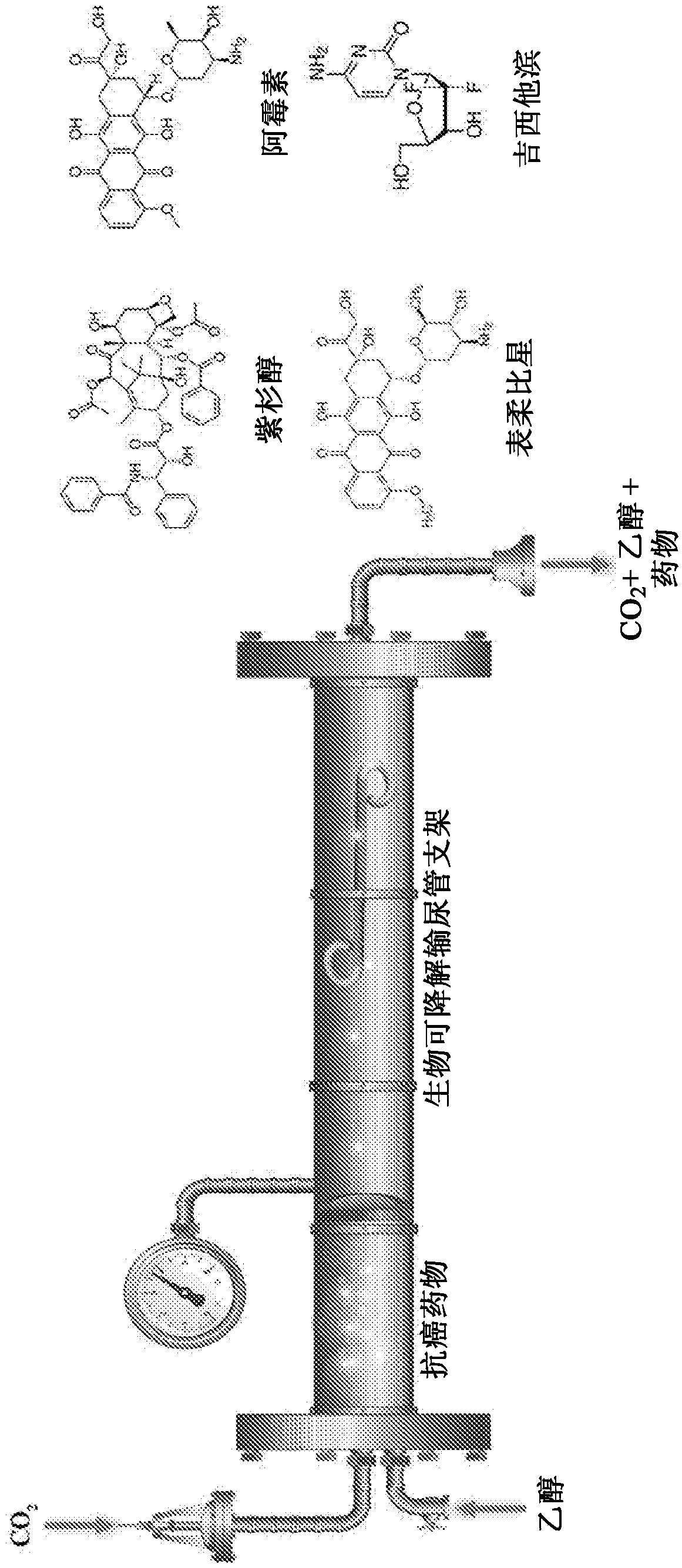
[0051] In one embodiment, hydrophobic anticancer drugs, paclitaxel, doxorubicin, epirubicin and / or gemcitabine, etc. are impregnated in the biodegradable ureteral stent by supercritical fluid technology.
[0052] In one embodiment, sodium alginate, gelatin, calcium chloride, chloroform, ethanol, and bismuth(III) subcarbonate were purchased from Sigma-Aldrich (Germany). Potassium dihydrogen ortho-phosphate (99.5%) and magnesium chloride hexahydrate (99%) were obtained from Riedel-de (Germany) obtained. Take TONE TM Polycaprolactone resin PCL 787 is commercially available in polymer form from Union Carbide Chemicals and Plastics Division, Bound Brook, New Jersey. Artificial urine (AUS), pacl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com