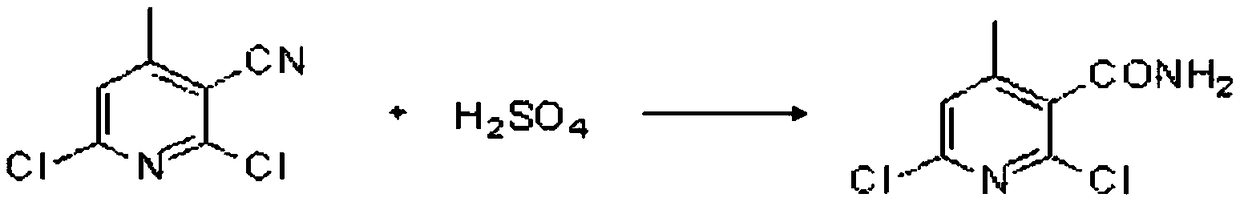
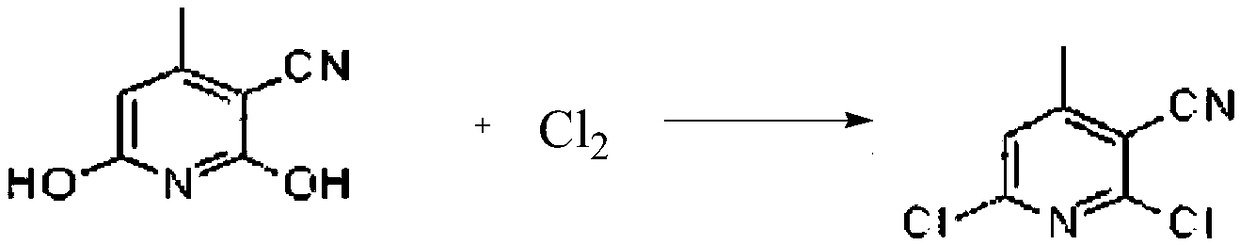Preparation method for nevirapine intermediate
A technology of nevirapine and intermediates, applied in the field of material synthesis, can solve the problems of high wastewater content, treatment of phosphorus oxychloride odor, etc., achieve short production cycle, reduce the discharge of three wastes, and achieve the effects of high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
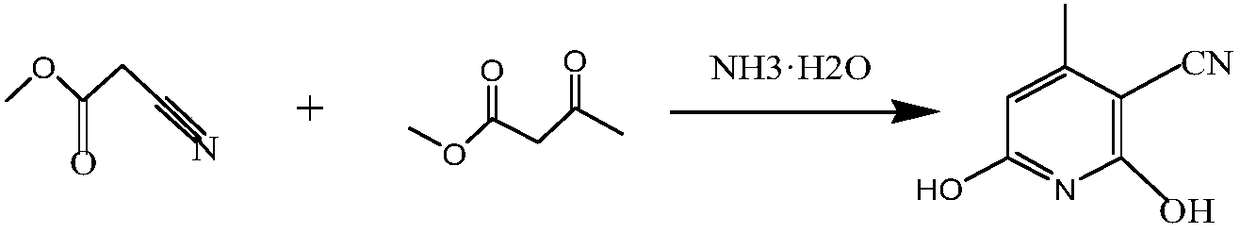
[0030] 1. The first step is to synthesize hydroxyl compounds:
[0031] Put in purified water and ammonia water, cool down to below 15°C after feeding, start to add 99% methyl cyanoacetate dropwise for about 1 hour (to generate cyanoacetamide), then add 99% diethyl methyl ester (methyl acetoacetate) dropwise , polycondensation reaction) the dropping time is about 1 hour, after the dropping is finished, keep warm at 10-15°C for 30 minutes, then raise the temperature to 20-25°C, keep warm at this temperature for 1 hour, then slowly raise the temperature to 70- 75°C and keep at this temperature for 3 hours. The system precipitated needle-like material, and cooled to 20°C for suction filtration, pumped the mother liquor into another kettle and raised the temperature to 60-65°C, adjusted the pH value to 7 with sulfuric acid, stirred for half an hour, and the pH remained unchanged, then cooled to 15°C for suction filtration, and the mother liquor into the sewage treatment station. ...
Embodiment 2
[0040] 1. The first step is to synthesize hydroxyl compounds:
[0041] Put in purified water and ammonia water, cool down to below 15°C after feeding, start to add 99% methyl cyanoacetate dropwise for about 1 hour (to generate cyanoacetamide), then add 99% diethyl methyl ester (methyl acetoacetate) dropwise , polycondensation reaction) the dropping time is about 1 hour, after the dropping is finished, keep warm at 10-15°C for 30 minutes, then raise the temperature to 20-25°C, keep warm at this temperature for 1 hour, then slowly raise the temperature to 70- 75°C and keep at this temperature for 3 hours. The system precipitated needle-like material, and cooled to 20°C for suction filtration, pumped the mother liquor into another kettle and raised the temperature to 60-65°C, adjusted the pH value to 7 with sulfuric acid, stirred for half an hour, and the pH remained unchanged, then cooled to 15°C for suction filtration, and the mother liquor into the sewage treatment station. ...
Embodiment 3
[0050] 1. The first step is to synthesize hydroxyl compounds:
[0051] Put in purified water and ammonia water, cool down to below 15°C after feeding, start to add 99% methyl cyanoacetate dropwise for about 1 hour (to generate cyanoacetamide), then add 99% diethyl methyl ester (methyl acetoacetate) dropwise , polycondensation reaction) the dropping time is about 1 hour, after the dropping is finished, keep warm at 10-15°C for 30 minutes, then raise the temperature to 20-25°C, keep warm at this temperature for 1 hour, then slowly raise the temperature to 70- 75°C and keep at this temperature for 3 hours. The system precipitated needle-like material, and cooled to 20°C for suction filtration, pumped the mother liquor into another kettle and raised the temperature to 60-65°C, adjusted the pH value to 7 with sulfuric acid, stirred for half an hour, and the pH remained unchanged, then cooled to 15°C for suction filtration, and the mother liquor into the sewage treatment station. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com