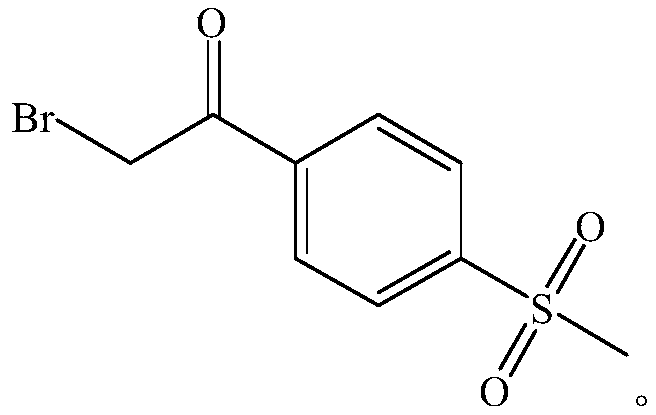
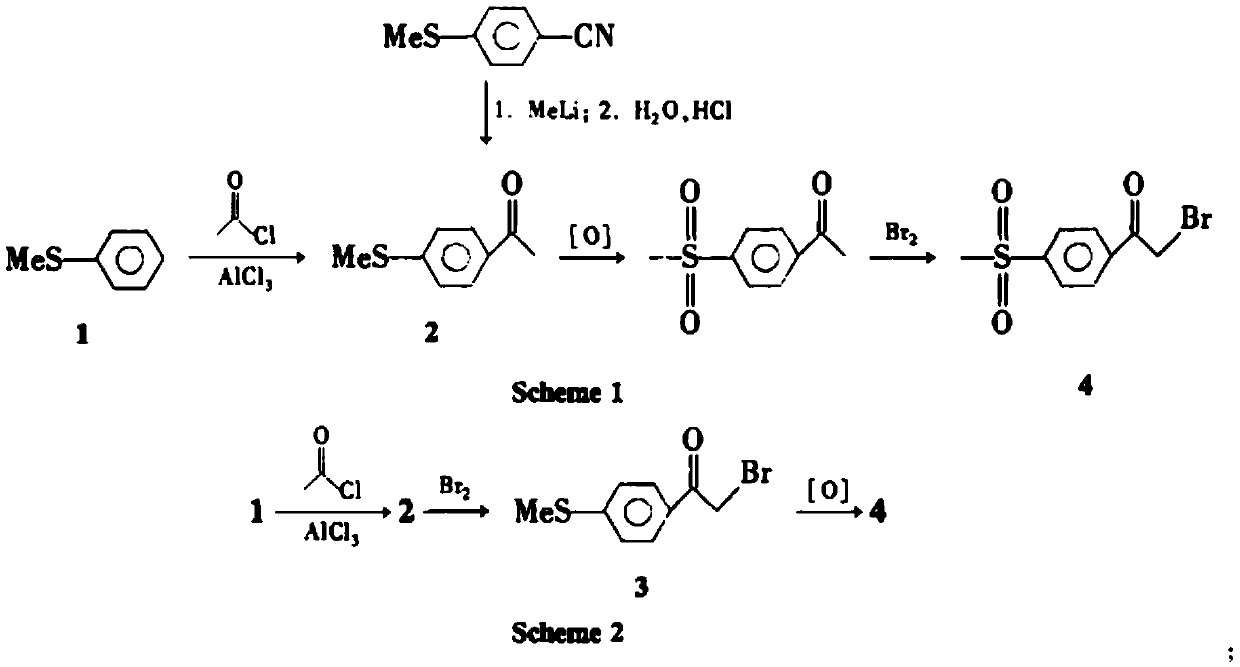
A kind of preparation method of 4-methanesulfonylacetophenone and α-bromo-4-methanesulfonylacetophenone
A kind of technology of methanesulfonyl acetophenone and acetophenone, applied in the preparation of 4-methanesulfonyl acetophenone and α-bromo-4-methanesulfonyl acetophenone, rofecoxib or erecoxib In the field of intermediates, it can solve the problems of waste of resources, easy miscibility, easy decomposition, etc., and achieve the effect of reducing sewage discharge, complying with environmental protection, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
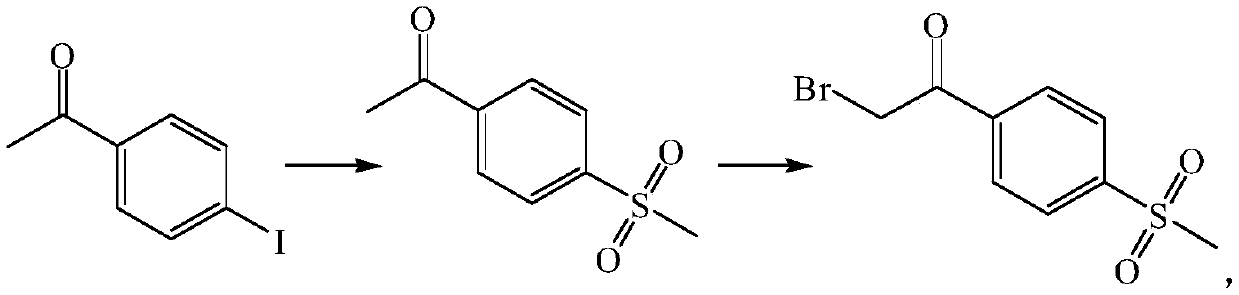
[0046] Embodiment 1 prepares 4-methylsulfonyl acetophenone
[0047] The route is as follows:
[0048]
example 1-1
[0050] 4-iodoacetophenone (24.6g), sodium methanesulfonate (15.3g), N,N-dimethyl-N-[2-(methacryloyloxy)ethyl]-1-butan Ammonium bromide (2.9g, formula I-5), potassium carbonate (20.7g) and cuprous iodide (1.9g) were added to 500ml of toluene, and heated to 110±2°C under nitrogen protection for 24 hours. After the reaction was completed, the reaction solvent was recovered by concentrating under reduced pressure. Add water and dichloromethane to dissolve, add activated carbon to absorb and decolorize, filter with a small amount of diatomaceous earth, separate layers, dry the dichloromethane layer with anhydrous sodium sulfate, concentrate to dryness, add ethanol for beating, filter, and dry to obtain 4- Methylsulfonyl acetophenone 18.1g, yield 91.3%, purity 98.2%.
example 1-2
[0052] 4-iodoacetophenone (24.6g), sodium methanesulfonate (20.4g), N,N-dimethyl-N-[2-(methacryloyloxy)ethyl]-1-benzyl Ammonium bromide (3.3g, formula I-1), cesium carbonate (65.1g) and cuprous iodide (1.9g) were added to 500ml of chlorobenzene, and heated to 110±2°C under nitrogen protection for 24 hours. After the reaction was completed, the reaction solvent was recovered by concentrating under reduced pressure. Add water and dichloromethane to dissolve, add activated carbon to absorb and decolorize, and filter with a small amount of diatomaceous earth. The layers were separated, and the dichloromethane layer was dried over anhydrous sodium sulfate and concentrated to dryness. Add ethanol for beating, filter and dry to obtain 18.8 g of 4-methylsulfonylacetophenone with a yield of 94.9% and a purity of 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com