Method for producing phospholipase D through recombinant escherichia coli
A technology for recombining Escherichia coli and Escherichia coli, applied in the fields of genetic engineering and microbial fermentation, can solve the problems of difficult to achieve high-density fermentation expression, cell lysis, and plasmid instability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1. Construction of recombinant bacteria TOP10 / pBADKP-PLD
[0039] Using the plasmid pUC57-par (containing the par region sequence of pSC101 shown in SEQ ID No.1) as a template, by primer 1 and primer 2 (Table 1), the par sequence was amplified by PCR; to contain the arabinose promoter P BAD The plasmid pBADK was used as a template, and the plasmid pBADK backbone was amplified by PCR with primers 3 and 4 (Table 1); the par sequence and pBADK backbone obtained by the above two amplifications were used as primers and templates, respectively, for POE-PCR, and the product was directly Transformed into E.coli DH5α, colony PCR verification and sequencing, to obtain the vector pBADKP; the plasmid pUC57-PLD containing the codon-optimized Streptomycesantibioticus PLD gene sequence shown in SEQ ID No.2 and the vector pBADKP were passed through Nco I and Xba I double digestion, T4 ligase ligation, transformed into E.coli TOP10 (recA - ), the colony PCR was verified and ...
Embodiment 2
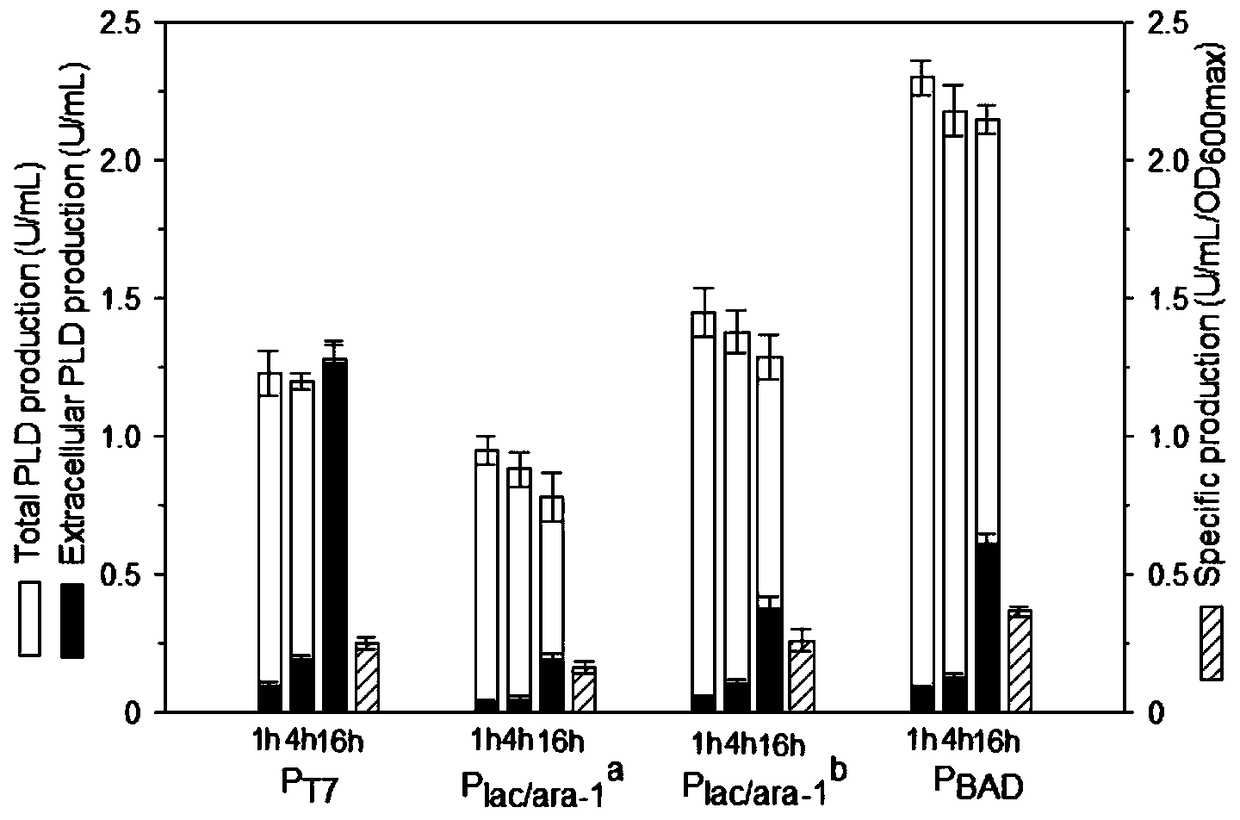
[0050] Example 2. Effect of promoter on phospholipase D expression.
[0051] Four promoters P T7 ,P lac / ara-1 a ,P lac / ara-1 b and P BAD It was used to investigate the expression of phospholipase D, and its activation strength decreased sequentially. where P lac / ara-1 a Partially activated by IPTG induction, P lac / ara-1 b Fully activated by IPTG and arabinose. Recombinant bacteria TOP10 / pBADKP-PLD was induced at 25°C for 16 hours, the results were as follows figure 1 shown. Under saturation induction, in the expression of PLD, P lac / ara-1 b and P BAD better than P T7 and P lac / ara-1 a , where P BAD Produces maximum PLD enzyme activity and specific yield. During the induction process, the PLD enzyme activities under all promoters almost reached the maximum after 1 hour of induction; after 16 hours of induction, the proportions of extracellular enzyme activities were 100%, 25%, 34% and 26%, respectively; process, the increase of extracellular enzyme activity ...
Embodiment 3
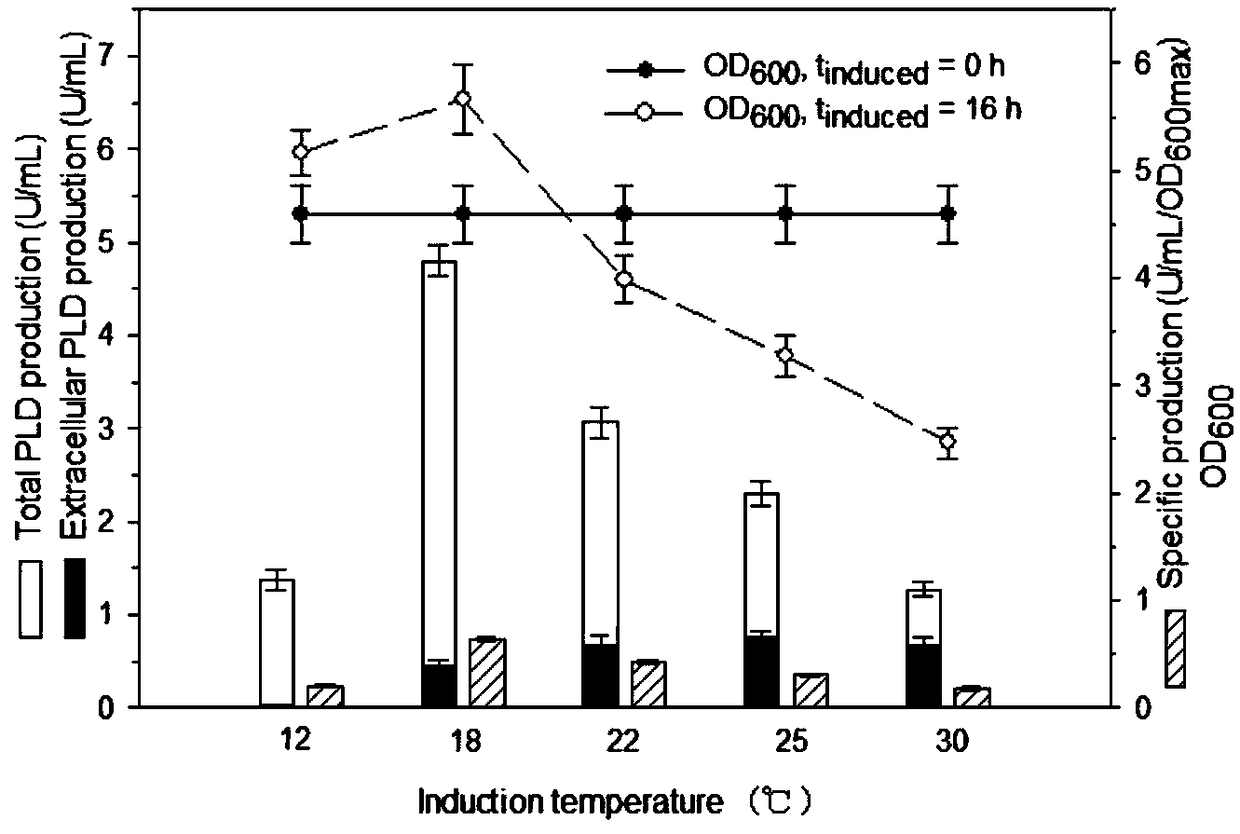
[0052] Example 3. Effect of Induction Temperature on Cell Growth and Phospholipase D Expression
[0053] In this experiment, TOP10 / pBADKP-PLD was cultured in TB medium at 37°C to OD 600 5 to 6, and then at 12°C, 18°C, 22°C, 25°C, 30°C and 37°C, respectively, to induce expression for 16 hours, and investigate the effect of induction temperature on the expression of PLD and biomass of TOP10 / pBADKP-PLD, the results are as follows figure 2 shown. Induction temperature significantly affected the expression of PLD and cell growth. The expression level was higher at 16-22°C, and the highest expression level appeared at 18°C. The specific productivity at 18°C was 3.8 times that at 30°C, and 92% of PLD appeared in the cell. Meanwhile, when the temperature was greater than 18°C, the expression of PLD decreased. The cell growth was significantly better at a moderate low temperature than at a high temperature, which reflected that a certain low temperature weakened the toxicity of P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com