Cationic liposome with aliphatic acid as membrane material and preparation method and application thereof
A cationic liposome and cationic lipid technology, applied in the field of biomedicine, can solve the problems of further improvement of targeting, low liposome encapsulation rate, complicated preparation process, etc., so as to improve blood microcirculation and enhance memory. and thinking ability, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 fatty acid
[0060] 30.0 mg of 10-hydroxyoctadecanoic acid (0.1 mmol) was dissolved in 5 mL of dichloromethane, 84.8 mg of Dess-Martin oxidizer (DMP, 0.2 mmol) and 33.6 mg of sodium bicarbonate (0.4 mmol) were added. The mixed solution was stirred overnight at room temperature (25±5°C). Add 10.6 mL of saturated sodium thiosulfate to the mixed solution and continue to stir for 2 hours, then extract with ethyl acetate, dry the organic phase over anhydrous magnesium sulfate, concentrate under reduced pressure, and separate and purify the crude product by flash column chromatography to obtain 10-carbonyl octadecadecane Carbonic acid;
[0061] Collect 30.0 mg of 10-carbonyl octadecanoic acid (0.1 mmol), dissolve it in 2 mL of methanol, then add 61.6 mg of ammonium acetate (0.8 mmol), and react at room temperature for 1.5 hours. Add 4.8 mg of sodium cyanoborohydride (0.1 mmol) to the mixed solution, stir for 2 days, then extract with ethyl ac...
Embodiment 2
[0065] The preparation of embodiment 2 fatty acids
[0066] 30.0 mg of 10-hydroxyoctadecanoic acid (0.1 mmol) was dissolved in 5 mL of chloroform, and 42.414 mg of Dess-Martin oxidizer (DMP, 0.1 mmol) and 8.4 mg of sodium bicarbonate (0.1 mmol) were added. The mixed solution was stirred overnight at room temperature. Add 5.772 mL of saturated sodium thiosulfate to the mixed solution and continue to stir for 3 hours, then extract with ethyl acetate, dry the organic phase over anhydrous magnesium sulfate, concentrate under reduced pressure, and separate and purify the crude product by flash column chromatography to obtain 10-carbonyl octadecadeca Carbonic acid;
[0067] Collect 30.0 mg of 10-carbonyl octadecanoic acid (0.1 mmol), dissolve it in 2 mL of isopropanol, then add 7.708 mg of ammonium acetate (0.1 mmol), and react at room temperature for 1.5 hours. Add 4.8 mg of sodium cyanoborohydride (0.1 mmol) to the mixed solution, stir for 2 days, then extract with ethyl acetate...
Embodiment 3
[0071] Example 3 Structural Identification of 10-Amino Octadecanoic Acid and Fatty Acid
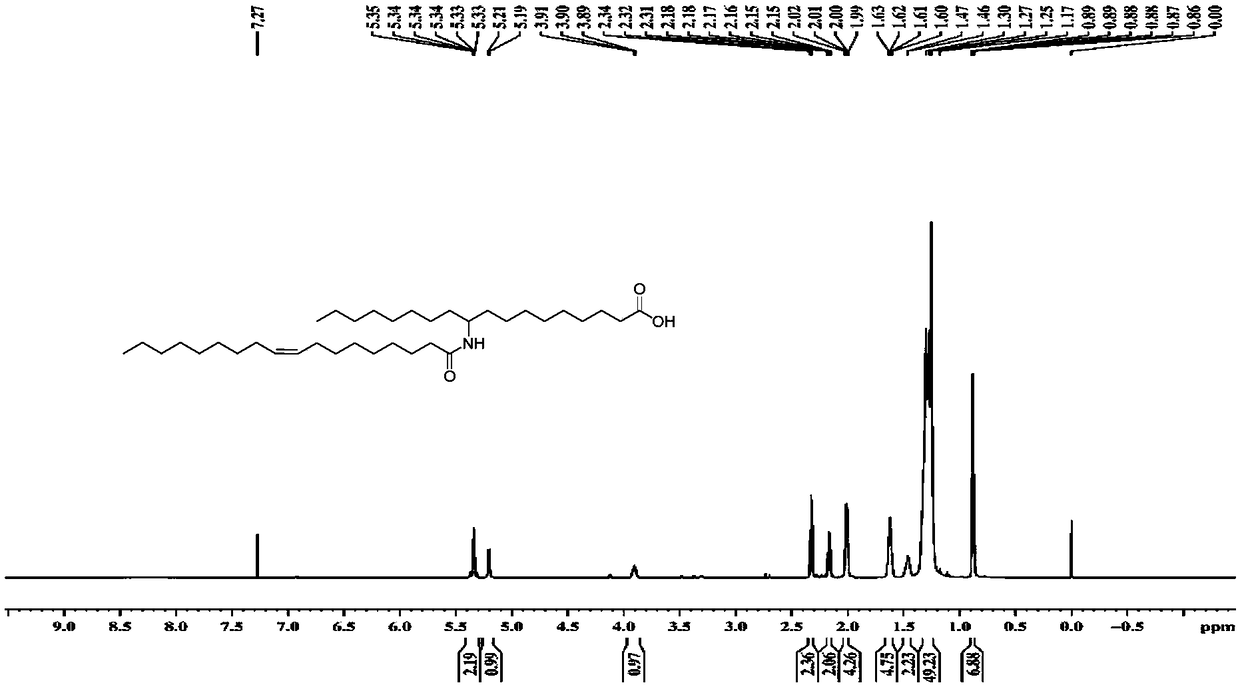
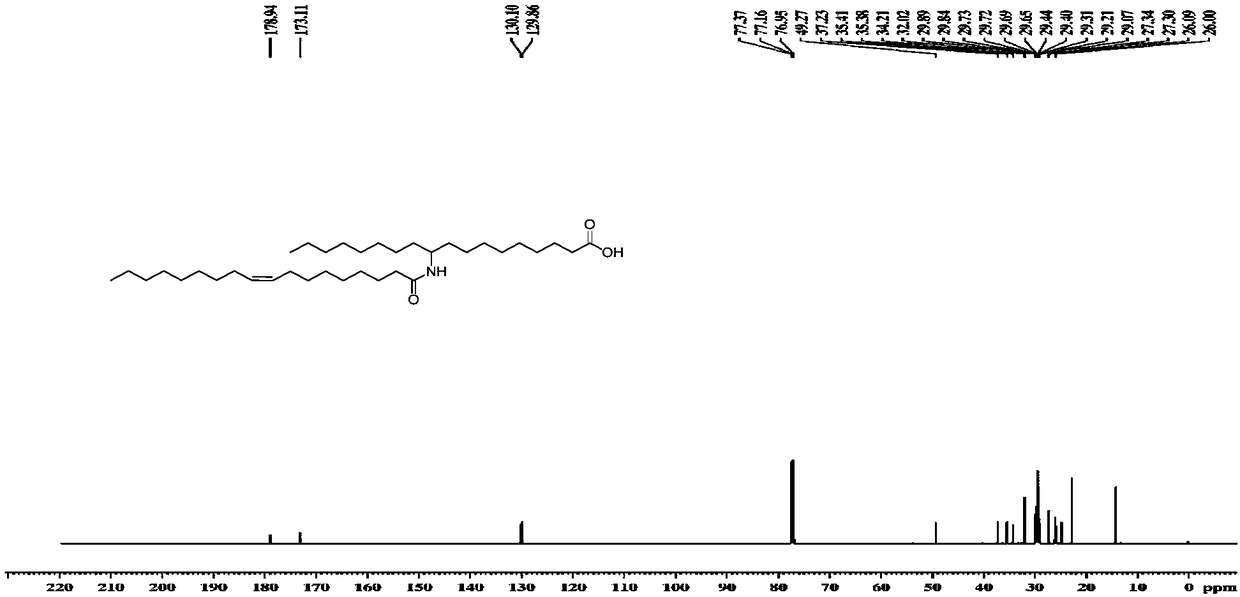
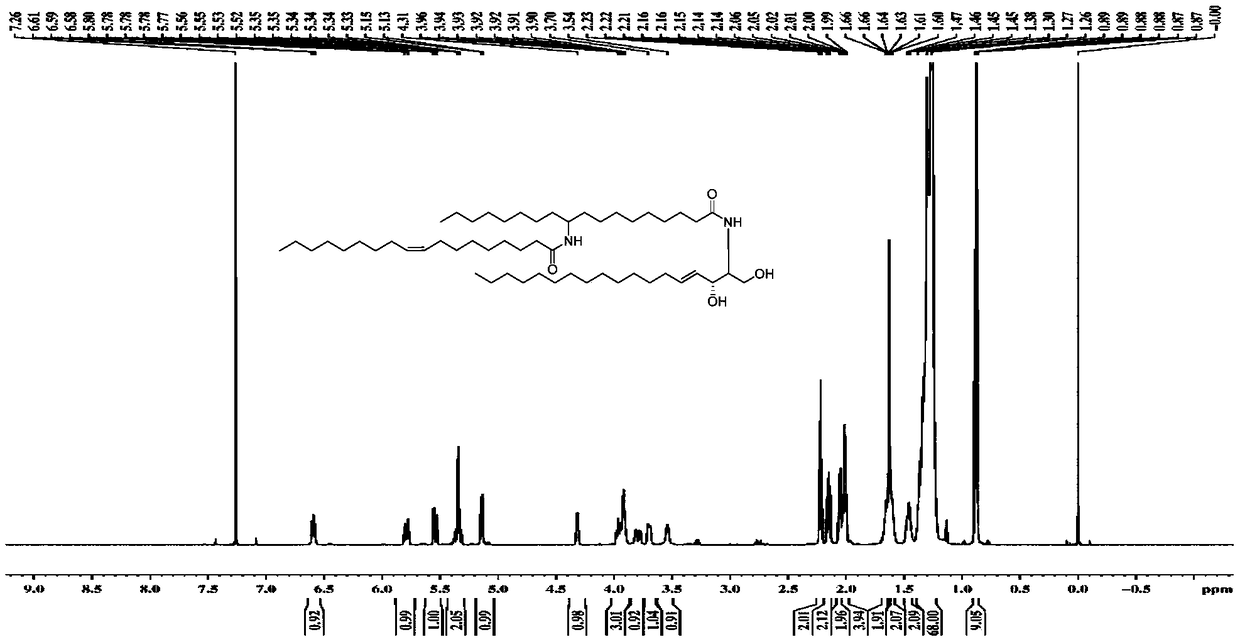
[0072] Utilize nuclear magnetic resonance spectrum to measure the 10-amino octadecanoic acid and fatty acid molecular structure that embodiment 1 prepares, obtain corresponding proton nuclear magnetic resonance spectrum figure and carbon nuclear magnetic resonance spectrum figure, as Figure 1-4 shown. The nuclear magnetic resonance spectroscopy determination method is a conventional technical means in the art, and will not be repeated here.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface potential | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com