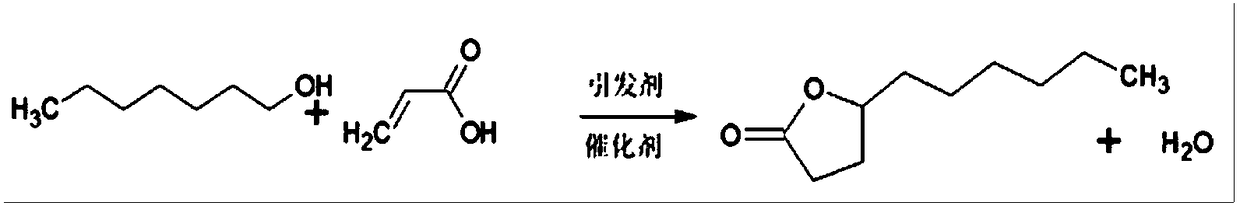
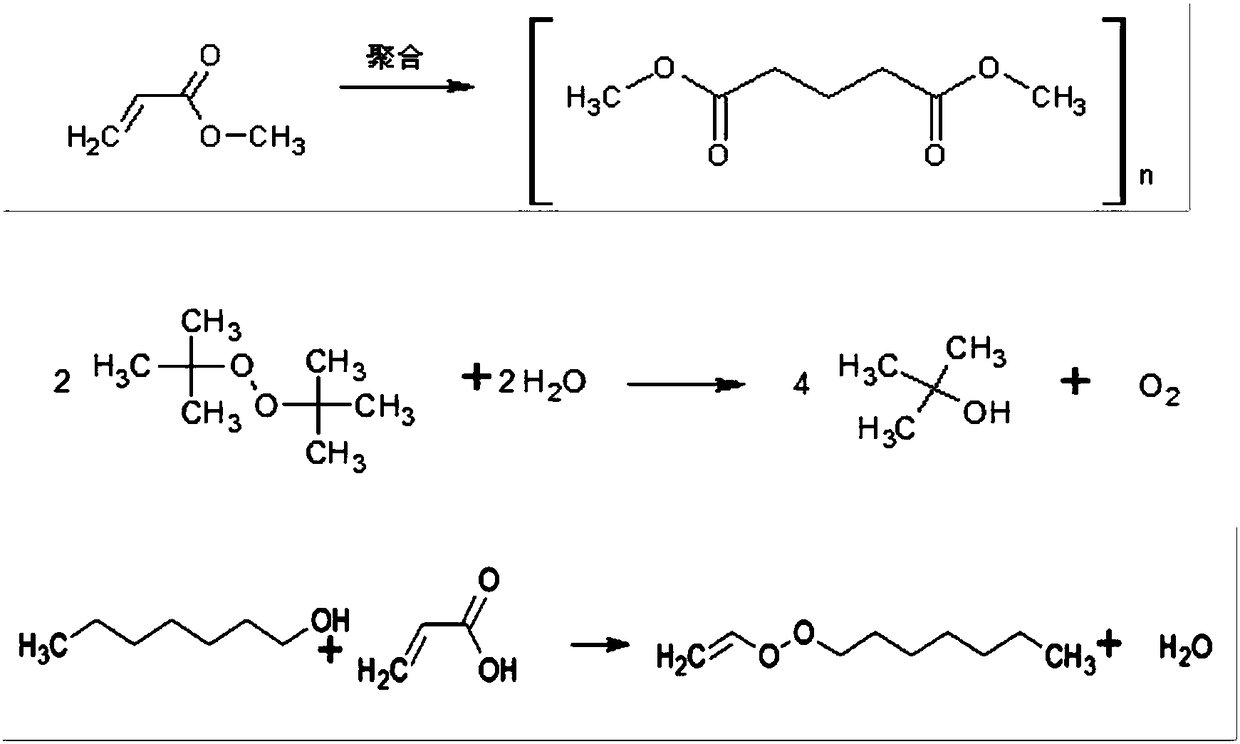
Method for compounding gamma-decalactone synthetic perfume through reactive distillation
A technology for the synthesis of gamma-decalactone and fragrances, which is applied in the direction of sustainable manufacturing/processing, chemical industry, climate sustainability, etc. It can solve the problems of insufficient aroma quality, complicated separation and purification process, and low reaction yield. , to achieve the effect of saving equipment investment, shortening reaction time and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for synthesizing perfume by reactive distillation, characterized in that: comprising the following steps:
[0038] (a) Add 120g of heptanol, 90g of methyl acrylate, 1g of catalyst sodium borohydride and 15g of initiator di-tert-butyl peroxide into a 500mL three-necked flask according to the proportion of ingredients, and turn on the electromagnetic stirrer and the integrated cooling and heat exchange machine , keeping the temperature at 20±5°C to obtain the ingredient mixture;
[0039] (b) Weigh again 360g of heptanol, add it to the 1500mL heater of the reactive distillation tower, turn on the electromagnetic stirrer and the integrated cooling and heat exchange machine, raise the temperature of the heater to 115-175°C and keep it warm, then put The batching mixture in the step (a) is sent into the reaction zone of the reactive distillation tower through the dripping pump; the temperature of the heater is controlled at 145-175°C during the dripping, and the drop...
Embodiment 2
[0047] The present embodiment prepares the synthetic perfume of gamma-decalactone according to the method of Example 1, the difference is that in step (a), the addition of each material is: heptanol 160g, di-tert-butyl peroxide 24g, Sodium borohydride 1g, methyl acrylate 95g;
[0048] In step (b), the amount of heptanol is weighed again to be 257g; the rest remain unchanged to obtain the gamma-decalactone product.
[0049] In this example, a total of 37 g of by-product tert-butanol and methanol were separated from the top of the tower, 128 g of the crude product of gamma-decalactone was separated from the bottom of the tower, and 13 g of low-boiling impurities and residual heptanol were collected to obtain gamma-decalactone 112g.
Embodiment 3
[0051] The present embodiment prepares the synthetic fragrance of gamma-decalactone according to the method of Example 1, the difference is that in step (a), the addition amount of each material is: heptanol 177g, di-tert-butyl peroxide 32g, Sodium borohydride 1.6g, methyl acrylate 75g;
[0052] In step (b), the amount of heptanol is weighed again to be 405g; the rest remain unchanged to obtain the gamma-decalactone product.
[0053] In this example, a total of 39 g of by-products tert-butanol and methanol were separated from the top of the tower, 103 g of crude product of gamma-decalactone was separated from the bottom of the tower, and 10 g of low-boiling impurities and residual heptanol were collected to obtain gamma-decalactone 89g.
[0054] The GC conditions for product detection are: chromatographic column HP-5 (30m×0.32mm×0.25μm); detector FID, temperature 280°C; sample injection: injection volume is about 0.2μl, split ratio 1:100, inlet temperature 250 °C; carrier ga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com