Polyurethane and preparation method of polyurethane and ROS (Reactive Oxygen Species)-responsive polyurethane drug-loading micelles
A drug-loaded micelle, polyurethane technology, applied in the polymer field, can solve the problems of premature drug release, wrong release timing, influence on tumor treatment effect, etc., and achieves simple and novel preparation method, enhanced drug and immunotherapy, and stimulated anti-tumor effect. Effects of tumor immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
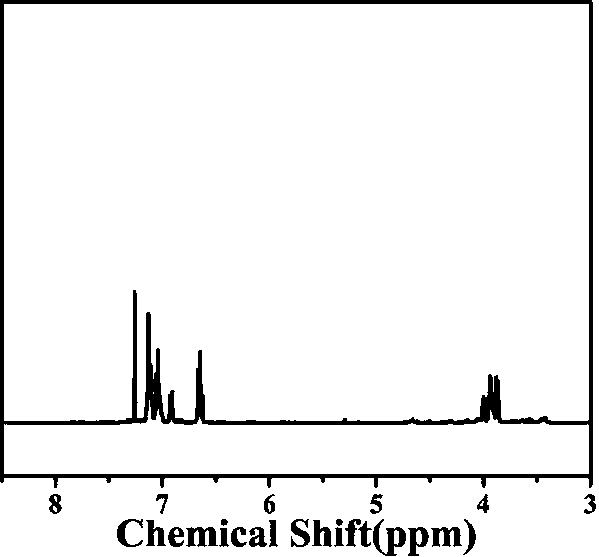
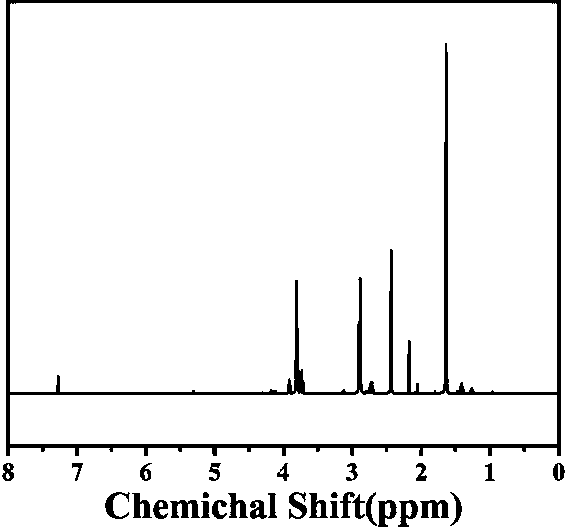
[0039] The preparation method of polyurethane includes:
[0040] S1. Dissolve 2g of 2,2'-bipyridine-4,4'-dicarboxylic acid in 50mL of ethanol. After heating to 60℃, add 5mL of concentrated sulfuric acid dropwise, keep it at 60℃ for 72 hours, and finally cool to room temperature. Add ethyl acetate and deionized water for extraction three times to obtain white solid ethyl 2,2'-bipyridine-4,4'-dicarboxylate (formula I),
[0041]
[0042] The reaction equation of S1 is,
[0043]
[0044] S2. Dissolve the white solid 2,2'-bipyridine-4,4'-dicarboxylate (formula I) prepared in 2g of S1 in methanol, and then add 0.5g of sodium borohydride at 65°C After refluxing for 48 hours, the solvent was evaporated to complete dryness, and then purified using a silica gel column. The eluent in the silica gel column was petroleum ether and ethanol. The volume fraction of petroleum ether: ethanol was 1:2, and finally The white solid 2,2'-bipyridine-4,4'-dimethanol (formula II) was prepared,
[0045]
[00...
Embodiment 2
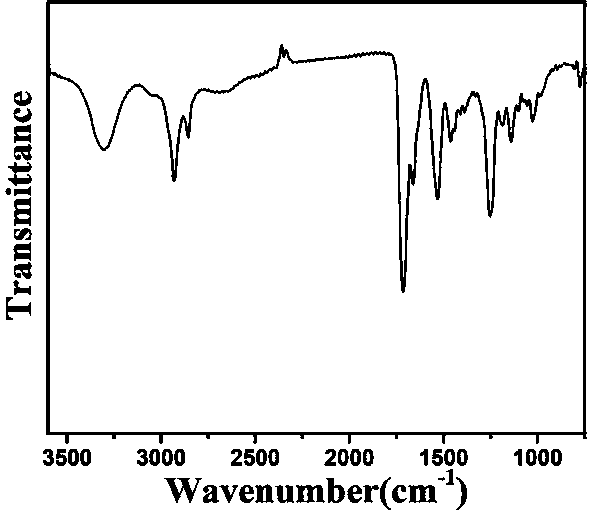
[0060] The preparation method of ROS-responsive polyurethane drug-loaded micelles, take 10mg of the light yellow polyurethane solid prepared in Example 1, and 0.3mg of hydrophobic drug dissolved in 2mL dimethyl sulfoxide solution, under vigorous stirring, slowly add 10mL dropwise Deionized water was then stirred for 3 hours. After the stirring, it was put into a dialysis bag with MW=3500. After dialysis for 2 days, the deionized water was replaced every four hours. The uncoated hydrophobic drug was removed by dialysis to obtain a stable ROS-responsive polyurethane drug-loaded micelles with near-infrared light-responsive photothermal therapy and drug synergy.
[0061] Such as image 3 , 4 , 5, the infrared spectrum, ultraviolet spectrum, fluorescence spectrum of the polyurethane drug-loaded micelles, the carbamate peak and the characteristic absorption peaks of the pyridine ring and benzene ring in the infrared spectrum prove the drug-loaded material The successful synthesis, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com