Cellulose endonuclease gene, protein and application thereof
A cellulose endonuclease and gene technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problem of few cellulose endonucleases, etc., and achieve the effect of a simple and efficient production and preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment provides an optimized artificial chemically synthesized endocellulase gene (EG), the specific sequence is shown in SEQ ID NO.1 in the sequence listing, and the protein sequence corresponding to the gene is shown in the sequence listing Shown in SEQ ID NO.2. The synthesized sequence has no sequence with a similarity of 30% in the NCBI database. It is a DNA optimized and synthesized according to the expression characteristics of Escherichia coli.
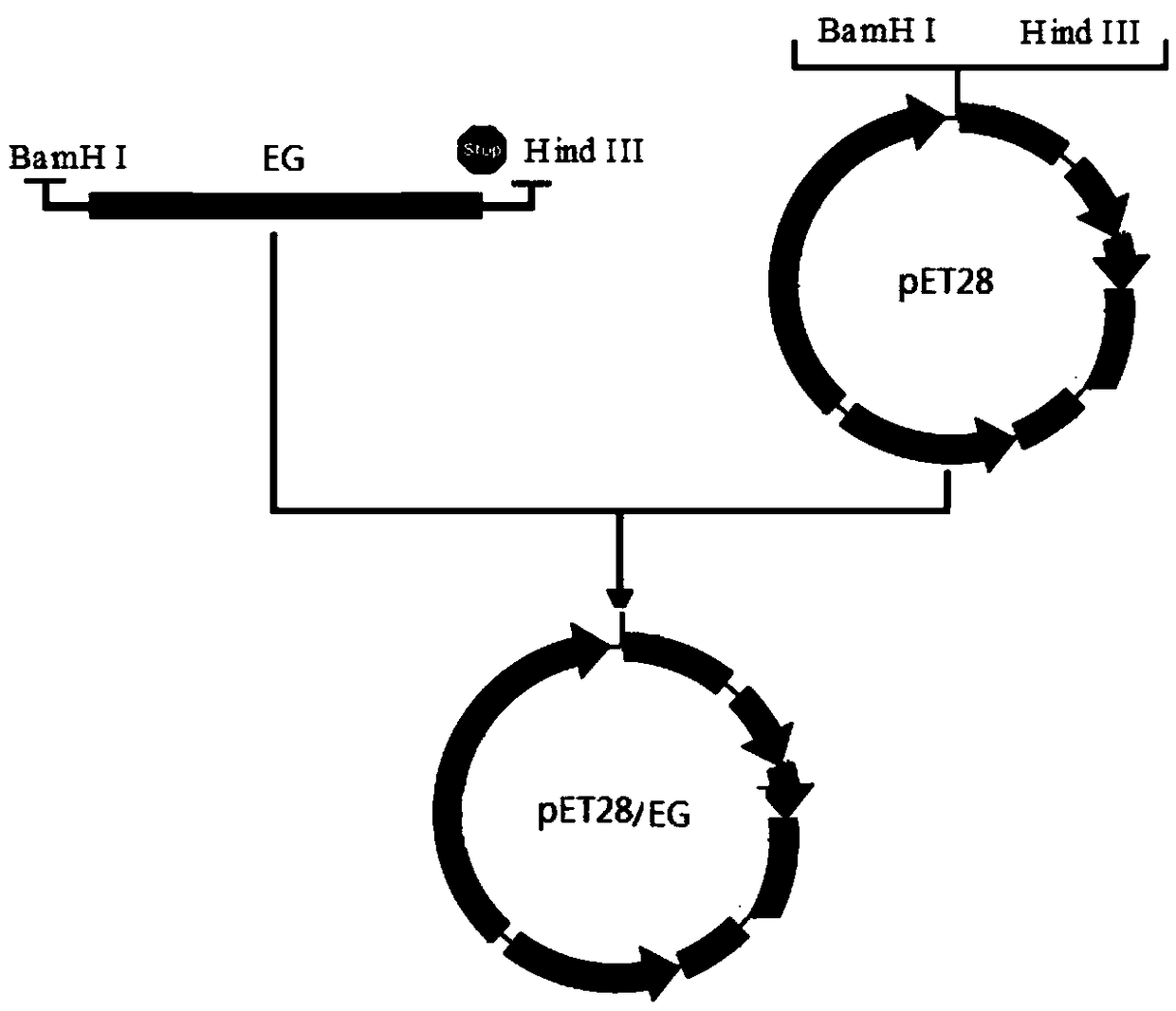
[0056] Connect the above-mentioned optimized gene into the E. coli expression vector pET28 to obtain the recombinant vector. The recombinant vector verified by sequencing above is heat-shocked and transformed into the competent cells of the E. coli expression strain, and the corresponding resistant LB plate is coated and kept at 37°C Cultivate in a constant temperature incubator for 12 hours, and screen transformants, wherein the pET28 / EG vector is constructed as figure 1 as shown, figure 1 It is a schematic d...
Embodiment 2
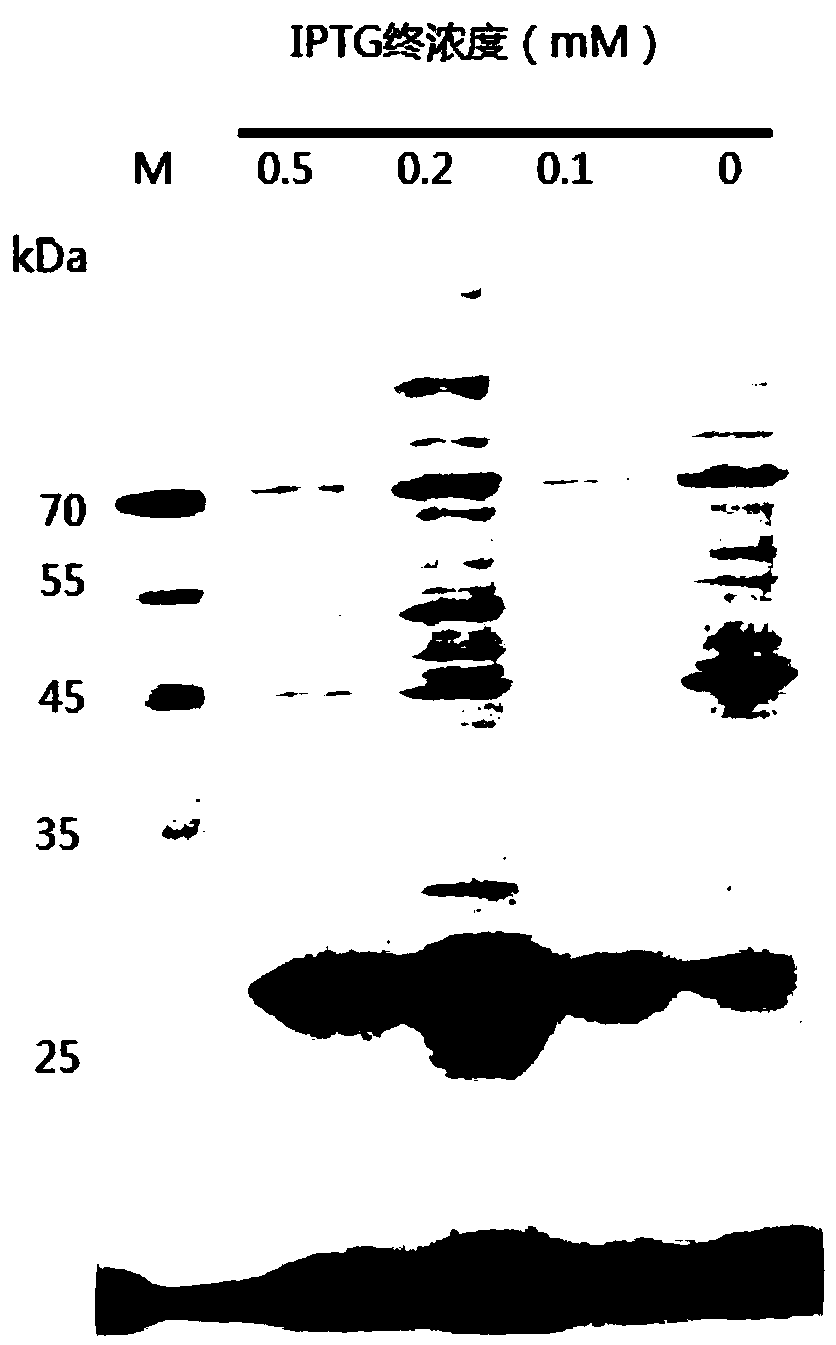
[0059] This embodiment provides a method for preparing protein, which specifically includes the following steps:
[0060] S1: Optimization of gene, construction of prokaryotic expression vector and transformation: Artificial chemical synthesis of the optimized mature cellulase gene was ligated to the pUC universal vector to obtain pUC / EG, pUC / EG was double-digested with BamHI and HindIII, and the obtained EG was obtained The fragment was then subcloned into the expression vector pET28 to obtain the recombinant expression vector pET28 / EG. The vector was constructed as follows: figure 1 shown. The main steps of pET28 / EG vector construction are as follows:
[0061] (1) Digest the recombinant vector pUC / EG with BamH I and Hind III to obtain the target fragment EG. The reaction system is as follows (all the endonucleases and buffers used are purchased from Dalian TAKARA Company):
[0062]
[0063] (2) Digest pET28 with BamH I and Hind III to obtain vector fragments. The reacti...
Embodiment 3
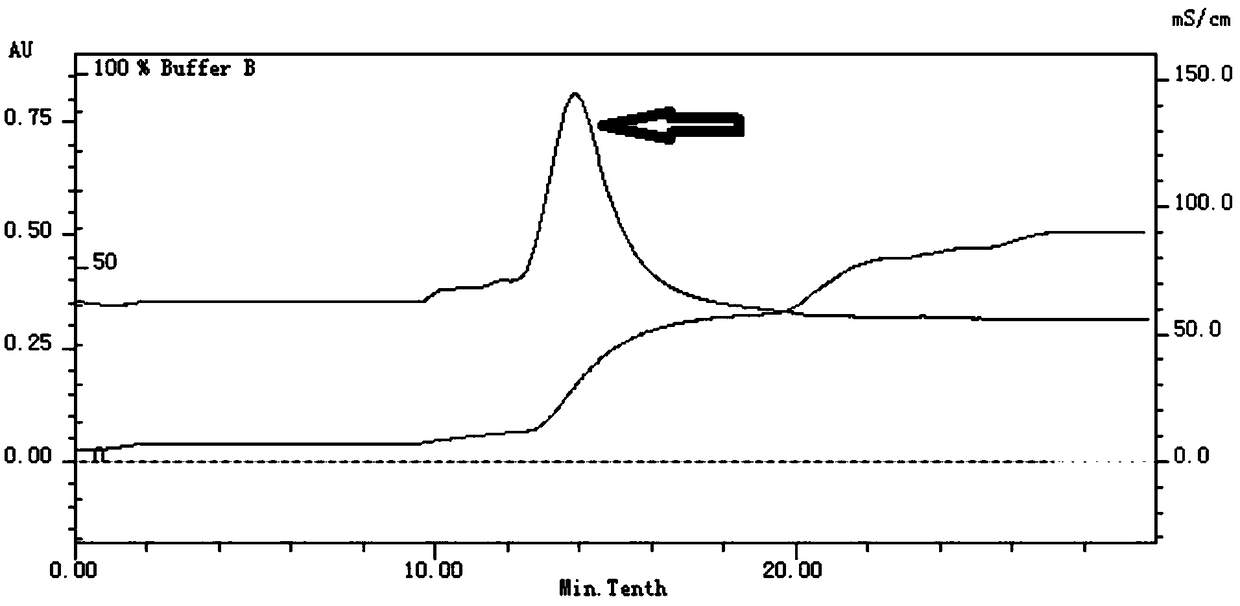
[0078] The invention uses high-performance liquid chromatography to detect the ability of sodium carboxymethylcellulose (CMC-Na) to be hydrolyzed by an endonuclease to produce a small amount of glucose to determine the enzyme activity. The specific method is as follows: 300 μg of purified recombinant endocellulase is added to the CMC-Na solution containing 1% at pH 4, and reacted for 4 hours at 40° C.; after the reaction, the sample is filtered with a 0.22 μm microporous membrane to Vials for liquid chromatography analysis. The liquid phase method is as follows: chromatographic column: Agilent amino column, 250×4.6mm, 5μm; mobile phase: acetonitrile:water=70:30 (volume ratio), flow rate: 1.0mL / min, injection volume: 10uL, column temperature : 35°C, detector: differential refractive index detector. HPLC results such as Figure 6 As shown, wherein the upper figure shows that CMC-Na does not detect the glucose peak without adding the recombinant endo-cellulase, and the lower fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com