Nickel-manganese-aluminum composite oxide catalyst for producing hydrogen by autothermal reforming of acetic acid
A technology of autothermal reforming and nickel-based catalysts, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, catalyst activation/preparation, etc., can solve problems such as catalyst deactivation, and promote Effects of dehydrogenation reaction, inhibition of acetic acid dehydration and ketonization reaction, and improvement of antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
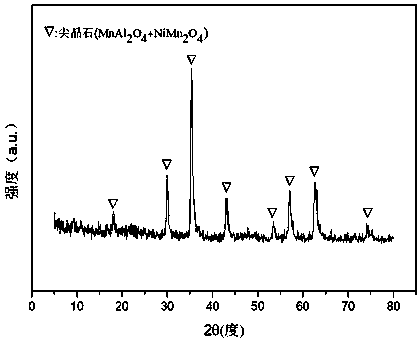
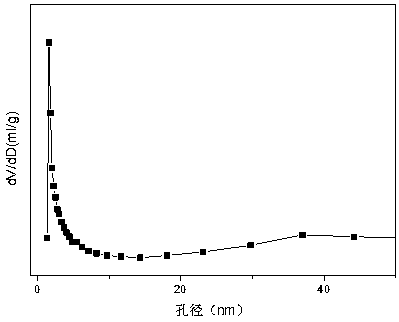
[0032] Weigh 2.335g of Ni(NO 3 ) 2 ·6H 2 O, 7.722g of Al(NO 3 ) 2 9H 2 O and 11.861g of Mn(NO 3 ) 2 (50 wt%), the above-mentioned nitrates were placed in a beaker together, then 61.75 mL of deionized water was added, stirred and dissolved with a magnetic stirrer, and solution #1 was prepared. Weigh 9.156g of NaOH and 1.516g of anhydrous Na 2 CO 3 , was added to 243.20ml of deionized water to prepare solution #2. Subsequent steps are the same as reference example 1, and obtain a typical structure such as attached figure 1 shown containing NiMn 2 o 4 and MnAl 2 o 4 Composite oxides with spinel structure, such as attached figure 2 Catalyst CUT-NMA-102 with mesoporous structure is shown. The molar composition of the catalyst is (NiO) 0.13 (MnO) 0.54 (AlO 1.5 ) 0.33 , and its weight composition is: nickel oxide is 15.0%, manganese oxide (MnO) is 58.8%, and aluminum oxide is 26.2%.
[0033] The catalyst CUT-NMA-102 has been tested on the autothermal reforming ac...
Embodiment 2
[0035] Weigh 2.035g of Ni(NO 3 ) 2 ·6H 2 O, 4.916g of Al(NO 3 ) 2 9H 2 O and 11.565g of Mn(NO 3 ) 2 (50 wt%), the above-mentioned nitrates were placed in a beaker together, then 52.42 mL of deionized water was added, stirred and dissolved with a magnetic stirrer, and solution #1 was prepared. Weigh 6.432g of NaOH and 1.065g of anhydrous Na 2 CO 3 , was added to 170.86ml of deionized water to prepare solution #2. Subsequent steps are the same as reference example 1, and obtain a typical structure such as attached figure 1 shown and attached figure 2 shown containing NiMn 2 o 4 and MnAl 2 o 4 Catalyst CUT-NMA-103 with composite oxide mesoporous structure with spinel structure. The molar composition of the catalyst is (NiO) 0.13 (MnO) 0.62 (AlO 1.5 ) 0.25 , and its weight composition is as follows: nickel oxide is 15.0%, manganese oxide (MnO) is 65.8%, and aluminum oxide is 19.2%.
[0036] The catalyst CUT-NMA-103 was tested for autothermal reforming activity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com