A nickel-chromium-manganese mesoporous composite oxide catalyst for hydrogen production by autothermal reforming of acetic acid
A composite oxide, nickel-chromium-manganese mesoporous technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, catalyst activation/preparation, etc., can solve the problem of catalyst structure instability, active group Solve problems such as easy oxidation and reduced catalyst activity, and achieve the effects of inhibiting acetic acid dehydration and ketonization reactions, improving activity and stability, and improving oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
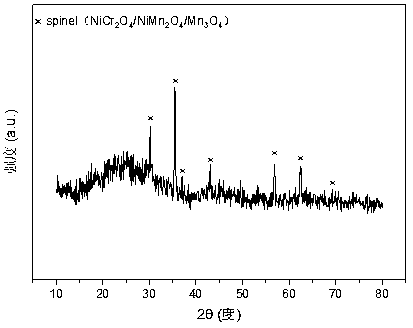
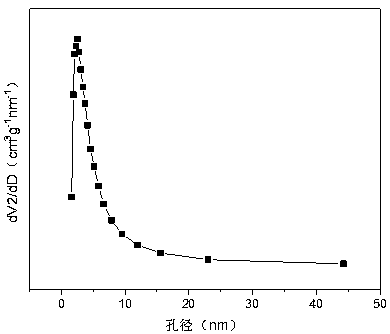
[0034] Weigh 4.781g of Cr(NO 3 ) 3 9H 2 O, 10.151g of Mn(NO 3 ) 2 (50wt%) and 2.372g of Ni(NO 3 ) 2 .6H 2 O, add 94.3ml of deionized water to make solution #1. Weigh 9.348g of NaOH and 1.548g of Na 2 CO 3 , adding 250ml of deionized water to prepare solution #2, the follow-up steps are the same as those in reference example 1, and a solution with a typical structure as attached is obtained. figure 1 shown containing NiMn 2 o 4 and NiCr 2 o 4 and Mn 3 o 4 and other spinel structures, such as attached figure 2 The molar composition of the mesoporous structure shown is (NiO) 0.17 (MnO) 0.58 (CrO 1.5 ) 0.25 The composite oxide CDUT-NM3C catalyst; The weight composition of this catalyst is: nickel oxide (NiO) content is 15.2%, manganese oxide (MnO) is 61.7%, chromium oxide (CrO 1.5 ) is 23.1%.
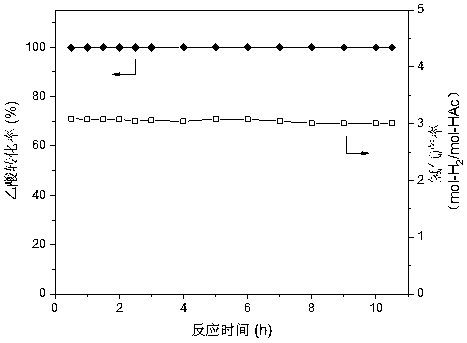
[0035] The catalyst CDUT-NM3C is tested by the autothermal reforming activity of acetic acid. When the reaction conditions are normal pressure, space velocity 30000ml / ...
Embodiment 2
[0037] Weigh 6.567g of Cr (NO 3 ) 3 9H 2 O, 8.810g of Mn(NO 3 ) 2 (50wt%) and 2.386g of Ni(NO 3 ) 2 .6H 2 O, add 96.3ml of deionized water to make solution #1. Weigh 9.452g of NaOH and 1.565g of NaOH 2 CO 3 , adding 250ml of deionized water to prepare solution #2, the follow-up steps are the same as those in reference example 1, and a solution with a typical structure as attached is obtained. figure 1 The crystal structure shown contains NiMn 2 o 4 and NiCr 2 o 4 and Mn 3 o 4 such as spinel, such as attached figure 2 (NiO) with mesoporous structure shown 0.17 (MnO) 0.50 (CrO 1.5 ) 0.33 Composite oxide CDUT-NM2C catalyst. The weight composition of this catalyst is: nickel oxide (NiO) is 15.3%, manganese oxide (MnO) is 53.5%, chromium oxide (CrO 1.5 ) is 31.2%.
[0038] The catalyst CDUT-NM3C was tested for activity of acetic acid autothermal reforming, the reaction conditions were normal pressure, space velocity 30000ml / (g-catalyst h), reaction temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com