Kit for detecting anti-MCV (mutation citrullinated vimentin) antibody and preparation method of kit
A technology of citrullination and vimentin, which is applied in the field of clinical immunoassay, can solve the problems of colloidal gold method such as low sensitivity and specificity, complicated detection steps, long reaction time, etc., to achieve improved sensitivity and specificity, narrow emission, affinity and high adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of a kit for detecting anti-mutant citrullinated vimentin antibodies
[0029] In the first step, a nitrocellulose membrane coated with anti-mutant citrullinated vimentin (MCV) was prepared
[0030] Take 30cm of nitrocellulose membrane and paste it on the self-adhesive liner, place it on the film scratcher, and coat the film with a spray volume of 1ul / cm according to the operating rules of the film scratcher; wherein the detection line is coated with a concentration of 0.5-1mg / ml MCV protein, the quality control line is coated with goat anti-mouse IgG antibody at a concentration of 1-2mg / ml, and dried at 37°C for 5 hours after coating;
[0031] The second step is to prepare a fluorescent microsphere conjugate pad coupled with mouse anti-human IgG antibody (quantum dot microspheres are used for fluorescent microspheres)
[0032] Take 2ml 0.5M PH8.0 Tris buffer in a clean glass bottle, add 100-200ug quantum dot microspheres and ultrasonically dispers...
Embodiment 2
[0039] The usage method of the kit prepared in embodiment 2 embodiment 1
[0040] 1. Reagent preparation and preparation:
[0041] 1. Place the kit and samples at room temperature (18-25°C) to equilibrate for at least 30 minutes.
[0042] 2. Quality control: Confirm that the chip card matches the batch number of the test card, and insert the fluorescence measuring instrument for quality control.
[0043] 2. Experimental operation:
[0044] 1. Open the test reagent and package, take out the test card and sample diluent and number them.
[0045] 2. Add sample
[0046] Take 20 μl of serum / plasma / whole blood sample, add it to the sample diluent, mix well, use a matching pipette to draw 60 μl into the sample hole of the test card, and start timing.
[0047] 3. Detection
[0048] Insert the test card into the instrument immediately after adding the test card for 10 minutes, click the "Test" button, the system will automatically read the card and display the test result. When the ...
Embodiment 3
[0049] Embodiment 3 The performance test of kit of the present invention
[0050] 1. Analytical sensitivity: the lowest detection limit is not higher than 0.5U / mL;
[0051] Analytical sensitivity is expressed in terms of detection limit, and the definition of analytical sensitivity is: it refers to the amount that can be distinguished from zero dose in a statistical sense. Repeat 20 times to measure the 0-value calibrator, calculate the mean (X) and standard deviation (SD), and the calculated concentration value of X+2SD is the analytical sensitivity of the kit. The analytical sensitivity of the kit of the present invention is 0.5U / mL.
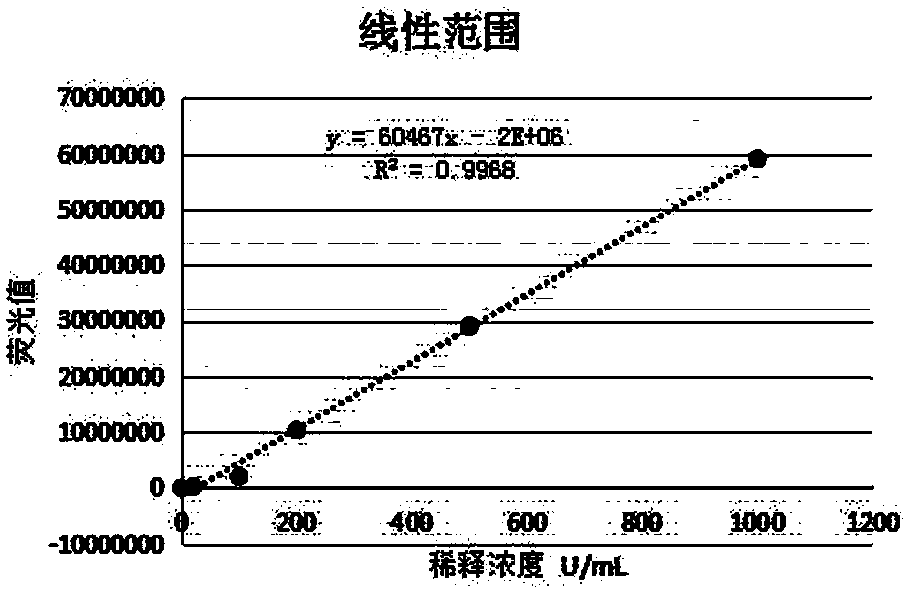
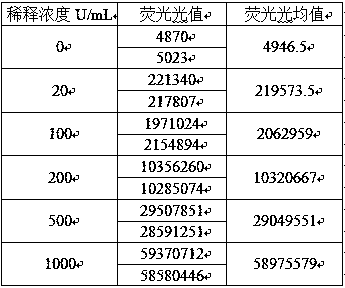
[0052] 2. Linear range: 0-1000 U / mL;
[0053] Dilute the high-value samples close to the upper limit of the linear range to at least 5 concentrations in a certain proportion, and the samples with low-value concentrations must be close to the lower limit of the linear range. The samples of each concentration were tested twice, the average va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com