Zinc phthalocyanine-doxorubicin conjugate with synergistic anticancer effects of photodynamic therapy and chemotherapy
A technology of zinc phthalocyanine and conjugates, applied in the field of drug preparation, can solve the problems of limited clinical application, high skin phototoxicity, lack of efficient combination drugs, etc., and achieve excellent amphiphilicity and high cancer cell uptake rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] 1. The preparation method of terminal dipeptide-phthalocyanine zinc compound (I), comprising the following steps:
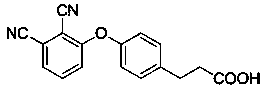
[0046] (1) With 1-[4-(2-carboxyethyl)phenoxy]zinc phthalocyanine and N-hydroxysuccinimide as reactants, the ratio of the two is 1:1.5-3, with N,N - Dimethylformamide as solvent, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride as dehydrating agent, under nitrogen protection, stirring reaction at -5-5°C After 1-2 hours, move to 20-35°C and continue to stir the reaction for 12-36 hours, then separate by column chromatography to obtain the carboxyl activated product of zinc phthalocyanine: ; Wherein, the consumption of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride needs 1.5-4mmol for every mmol of zinc phthalocyanine reactant;
[0047] (2) The carboxyl activated product of zinc phthalocyanine and glycine-proline dipeptide prepared in step (1) are used as reactants, and the feeding ratio of the two is 1:1.5-2, and N,N-dimethyl formazan...
Embodiment 1
[0082] Compound (Ⅱ) The preparation method is:
[0083] (1) First prepare the carboxyl activated product of zinc phthalocyanine whose structure is shown in the following formula:
[0084] ;
[0085] With 1-[4-(2-carboxyethyl)phenoxy]zinc phthalocyanine (60 μmol) and N-hydroxysuccinimide (120 μmol) as reactant, DMF as solvent (5ml), in 1-ethyl In the presence of 3-(3-dimethylaminopropyl)carbodiimide hydrochloride (180 μmol) and nitrogen protection, the reaction was stirred at 5°C for 1.5 hours, moved to room temperature and continued to stir for 20 hours, and the reaction was carried out by thin-layer chromatography. Monitor the endpoint of the reaction. After the reaction is complete, the reaction solution is concentrated by rotary evaporation, passed through a silica gel column, and the first blue band is collected with a mixed solvent of dichloromethane / tetrahydrofuran (volume ratio: 15:1) as the eluent, and then rotary evaporated to dryness after collection, and dried...
Embodiment 2
[0090] Preparation of compound (Ⅲ): ;
[0091] (1) to (Ⅶ) (60μmol) and N-hydroxysuccinimide (120μmol) as reactant, with DMF as solvent (5ml), in 1-ethyl-(3-dimethylaminopropyl) carbodiimide In the presence of hydrochloride (180 μmol) and under the protection of nitrogen, the reaction was stirred at 5°C for 1.5 hours, moved to room temperature and continued to stir for 20 hours, and the end of the reaction was monitored by thin-layer chromatography. After the reaction is complete, the reaction solution is concentrated by rotary evaporation, passed through a silica gel column, and the first blue band is collected with a mixed solvent of dichloromethane / tetrahydrofuran (15:1 by volume) as the eluent, and then rotary evaporated to dryness after collection, and dried in vacuum Finally, a blue powder is obtained with a yield of 70-80%.
[0092] (2) Using the carboxyl activated product of zinc phthalocyanine (40 μmol) and threonine-serine-glycine-proline tetrapeptide (60 μmol) co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com