Kit for detecting mycoplasma pneumonia
A technology of Mycoplasma pneumoniae and a kit, which is applied to measurement devices, instruments, disease diagnosis and other directions, can solve the problems of prone to cross-reaction, high requirements on culture conditions, and complicated operations, and achieves the advantages of spectrophotometric detection, shortened reaction time, The effect of increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
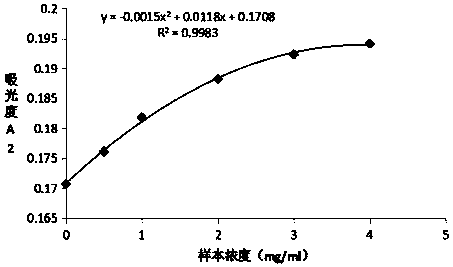
[0043] Preparation and detection of Mycoplasma pneumoniae P1 latex-enhanced immunoturbidimetric assay kit
[0044] (1) Preparation of reagent R1:
[0045] Weigh 23.83g of Hepes, 9g of NaCl, 15g of PEG8000, 0.5g of sodium azide, and 5g of bovine serum albumin, adjust the pH to 7.4, and dissolve in 1L to obtain reagent R1.
[0046] (2) Preparation of reagent R2:
[0047] The first step: with the MES buffer solution of 0.02M / L, the Mycoplasma pneumoniae cell membrane component (Mycoplasma pneumoniae is donated by the Chinese Academy of Medical Sciences, and the cell membrane component is obtained by processing) is diluted to a concentration of 2 mg / ml of the Mycoplasma pneumoniae cell membrane component dilution; Styrene latex microspheres (136nm) were washed 3 times with distilled water, and the supernatant was removed.
[0048] Step 2: Dilute the washed polystyrene latex microspheres to a mass concentration of 1% with MES buffer solution having a pH of 5.3 and a concentration...
Embodiment 2
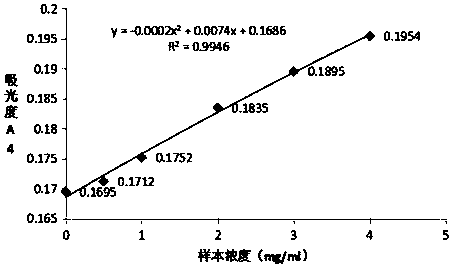
[0053] Preparation and detection of Mycoplasma pneumoniae P116 latex-enhanced immunoturbidimetric assay kit
[0054] (1) Preparation of reagent R1:
[0055] Weigh 23.83g of Hepes, 9g of NaCl, 15g of PEG8000, 0.5g of sodium azide, and 5g of bovine serum albumin, adjust the pH to 7.4, and dissolve in 1L to obtain reagent R1.
[0056] (2) Preparation of reagent R2:
[0057] Step 1: Dilute the human mycoplasma pneumoniae antigen P116 with 0.02M / L MES buffer solution to a concentration of 2 mg / ml of mycoplasma pneumoniae antigen P116 dilution; the polystyrene latex microspheres are washed with distilled water.
[0058] Step 2: Dilute the washed polystyrene latex microspheres to a mass concentration of 1% with MES buffer solution having a pH of 6.0 and a concentration of 0.02M / L. Add 0.01g of EDC to 1L of the above-mentioned latex dilution, stir and react at room temperature for 30min, centrifuge at a speed of 15000rpm for 15min after the reaction to remove the supernatant, resusp...
Embodiment 3
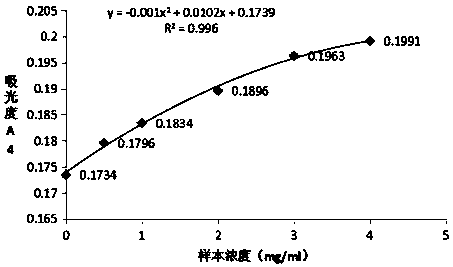
[0063] Preparation and detection of Mycoplasma pneumoniae P30 latex-enhanced immunoturbidimetric assay kit
[0064] (1) Preparation of reagent R1:
[0065] Weigh 23.83g of Hepes, 9g of NaCl, 15g of PEG8000, 0.5g of sodium azide, and 5g of bovine serum albumin, adjust the pH to 7.4, and dissolve in 1L to obtain reagent R1.
[0066] (2) Preparation of reagent R2:
[0067] Step 1: Dilute the human mycoplasma pneumoniae antigen P30 with 0.02M / L MES buffer to a concentration of 2mg / ml mycoplasma pneumoniae antigen P30 dilution; polystyrene latex microspheres (136nm) are washed with distilled water.
[0068] Step 2: Dilute the washed polystyrene latex microspheres to a mass concentration of 1% with MES buffer solution having a pH of 5.0 and a concentration of 0.02M / L. Add 0.01g of EDC to 1L of the above-mentioned latex dilution, stir and react at room temperature for 30min, centrifuge at a speed of 15000rpm for 15min after the reaction to remove the supernatant, resuspend with 0.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com