Method for synthesizing zinc methionine chelates
A technology of zinc methionine and synthesis method, applied in the field of synthesis of zinc methionine chelate, can solve the problems of low yield, waste discharge, difficult reaction, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
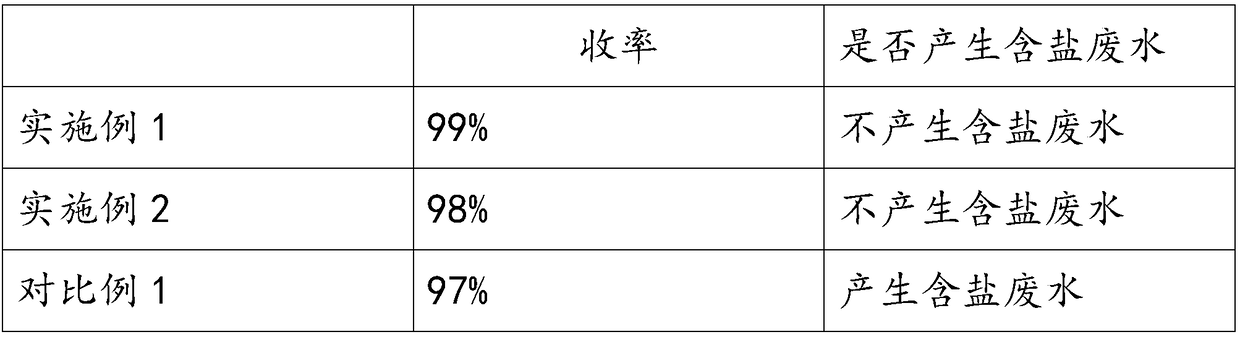
[0014] Weigh 300g of glycerin and 100g of water, put them into the reaction vessel, stir and mix, add 30g of methionine, 11.6g of zinc carbonate and 0.5g of sodium salicylate successively during the stirring process, heat to boiling under normal pressure, and the boiling temperature is at At about 120 degrees Celsius, react under the reflux of the solution for 6 hours. After the reaction, filter while hot to obtain a filter cake. Rinse the filter cake twice with an appropriate amount of water. After the filter cake is dried and pulverized, 36g of zinc methionine is obtained, with a yield of 99%. , the obtained methionine zinc chelate structure formula is: [CH 3 SCH 2 CH 2 (NH 2 )CHCOO] 2 Zn.
Embodiment 2
[0016] Weigh 200g of glycerin and 200g of water, put them into the reaction vessel, stir and mix, add 46g of methionine, 10.7g of zinc oxide and 4g of sodium oxalate successively during the stirring process, under the conditions of 3 standard atmospheric pressures and a temperature of 170°C in a closed state React for 1 hour. After the reaction finishes, filter while hot to obtain the filter cake. Rinse the filter cake twice with an appropriate amount of water. After the filter cake is dried and pulverized, 46g of methionine is obtained with a yield of 99%. The structural formula of the zinc methionine chelate compound is : [CH 3 SCH 2 CH 2 (NH 2 )CHCOO] 2 Zn.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com