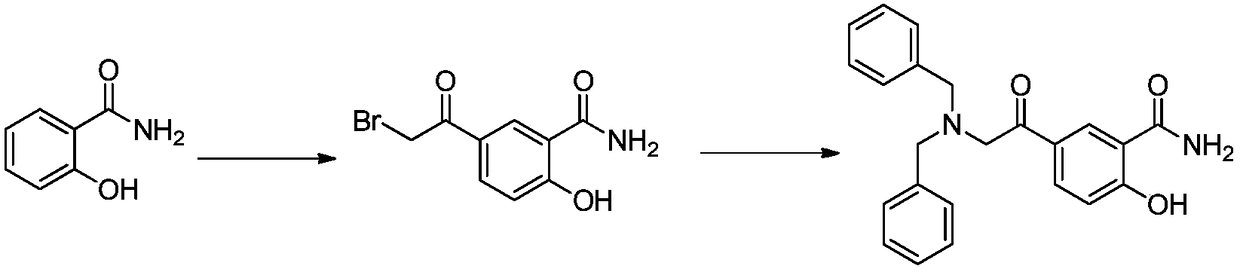
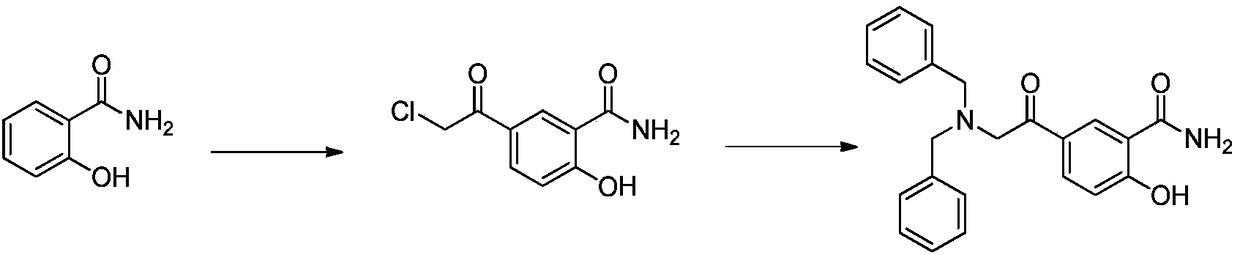
Synthetic method of 5-N,N-dibenzylamine acetyl salicylamide
A technique for the synthesis of dibenzylamine acetylsalicylamide and its synthesis method, which is applied in the field of synthesis of labetalol intermediate 5-N,N-dibenzylamine acetylsalicylamide, and can solve the problem of affecting the yield, many by-products, It is not easy to preserve and other problems, and achieves the effects of saving production costs, mild reaction conditions, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 500L reactor, add salicylamide (70Kg, 510mol), benzaldehyde (65Kg, 612mol), p-toluenesulfonic acid (1Kg, 5.8mol) and 140L toluene respectively, and the reaction solution is raised to 110°C and stirred at reflux for 6 ~8 hours; then the reaction solution was cooled to 0°C~5°C for crystallization, filtered, and dried to obtain 97.5Kg of 2-phenyl-3,4-dihydro-2H-benzo[1,3]oxazine- 4-Kone was used as intermediate I with a yield of 85%.
Embodiment 2
[0030] Add 2-phenyl-3,4-dihydro-2H-benzo[1,3]oxazin-4-one (90Kg, 400mol) and 270L nitromethane into a 500L reactor, then cool down to 0°C At ~10°C, add aluminum trichloride (107Kg, 800mol) in batches; after the addition is complete, add bromoacetyl chloride (69.3Kg, 440mol) dropwise at this temperature, and after the addition, warm to room temperature and stir for 6-8 hours. Then, the reaction liquid was introduced into another reaction kettle to quench in ice water, crystallized, filtered, and dried to obtain 125Kg of 2-phenyl-3,4-dihydro-2H-benzo[1,3]oxazine-4 -Bromoethyl ketone as intermediate II, the yield is 90%.
Embodiment 3
[0032] Add 2-phenyl-3,4-dihydro-2H-benzo[1,3]oxazine-4-bromoethanone (115Kg, 331mol), dibenzylamine (78.4Kg, 397mol ), triethylamine (40Kg, 397mol), potassium iodide (10Kg, 60mol) and 300L tetrahydrofuran, the temperature of the reaction solution was lowered to 10°C-20°C and stirred for 6-8 hours. After the reaction, filter, then put the filter cake into a solution of 300L tetrahydrofuran / water = 1:1, use concentrated hydrochloric acid to adjust the pH value to 1, keep warm at 10°C-20°C and react for 12-14 hours, then add sodium bicarbonate Adjust the pH value to 5-6, filter, and dry to obtain 105 Kg of 5-N,N-dibenzylamine acetylsalicylic amide as the final product, with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com