Method for preparing drug-carrying polyester polymer/biological ceramic bone repairing scaffold through low-temperature 3D printing technology as well as product and application thereof
A 3D printing and bioceramic technology, applied in the field of biomedical materials, can solve the problems of large influence of bioceramic melting temperature, limitation of bioceramic ratio, and affecting material processing performance, etc., to achieve good uniformity, maintain biological activity, and wide clinical application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
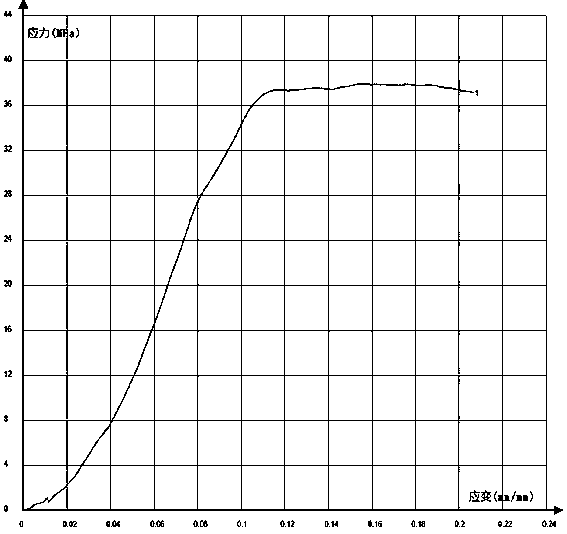
[0030] Weigh 4.45g of PLGA (Mw=20W), 0.5g of nHA powder, and 0.05g of vancomycin hydrochloride, add them to 10mL of hexafluoroisopropanol, and stir mechanically at room temperature for more than 24 hours to fully dissolve and mix the materials uniformly, and prepare a 3D Print "ink". The 3D printer is 3D-Bioplotter®, put the above "ink" in the barrel of the 3D printer, select the 22# discharge needle, and set the printing parameters: each layer is printed in parallel, the gap is 0.2mm, and the Z-axis direction rises each time 0.2mm, vertical cross-stacking between layers, extrusion speed set at 1mm / s, receiving platform temperature at -10°C, print size as length*width*height=6mm*6mm*4mm cube. After the scaffold was printed, it was freeze-dried for 48 hours, and then placed in a vacuum oven at 60°C. The compressive strength of the material within the range of elastic deformation is 37.95MPa.
[0031] as attached figure 1 In the SEM image of the printed product in this embodi...
Embodiment 2
[0033] Weigh 2.45g of PLGA (Mw=20W), 2.5g of nHA powder, and 0.05g of vancomycin hydrochloride, add it to 10mL of hexafluoroisopropanol, and stir mechanically at room temperature for more than 24 hours to fully dissolve and mix the materials, and prepare a 3D Print "ink". The 3D printer is 3D-Bioplotter®, put the above "ink" in the barrel of the 3D printer, select the 22# discharge needle, and set the printing parameters: each layer is printed in parallel, the gap is 0.2mm, and the Z-axis direction rises each time 0.2mm, vertical cross-stacking between layers, extrusion speed set at 1mm / s, receiving platform temperature at -10°C, print size as length*width*height=6mm*6mm*4mm cube. After the scaffold was printed, it was freeze-dried for 48 hours, and then placed in a vacuum oven at 60°C. The compressive strength within the elastic deformation range of the material is 10.35MPa.
Embodiment 3
[0035] Weigh 3.95g of PLGA (Mw=10W) and PCL (Mw=8W) at a mass ratio of 5:5, 1g of nHA powder, and 0.05g of vancomycin hydrochloride, add it to 10mL of hexafluoroisopropanol, and stir mechanically at room temperature for 24h Above, the materials are fully dissolved and mixed evenly to prepare the 3D printing "ink". The 3D printer is 3D-Bioplotter®, put the above "ink" in the barrel of the 3D printer, select the 22# discharge needle, and set the printing parameters: each layer is printed in parallel, the gap is 0.2mm, and the Z-axis direction rises each time 0.2mm, vertical cross-stacking between layers, extrusion speed set at 1mm / s, receiving platform temperature at -10°C, print size as length*width*height=6mm*6mm*4mm cube. After the scaffold was printed, it was freeze-dried for 48 hours, and then placed in a vacuum oven at 60°C. The compressive strength of the material within the range of elastic deformation is 24.08MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com