Preparation method of rifabutin intermediate
A technology for rifabutin and intermediates, which is applied in the field of preparation of rifabutin intermediates, can solve problems such as uneven properties, unfavorable industrialization, and difficulty in stratification, and achieves easy preservation, easy industrialization, and non-corrosive preservation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of preparation of rifabutin intermediate 3-bromorifamycin S
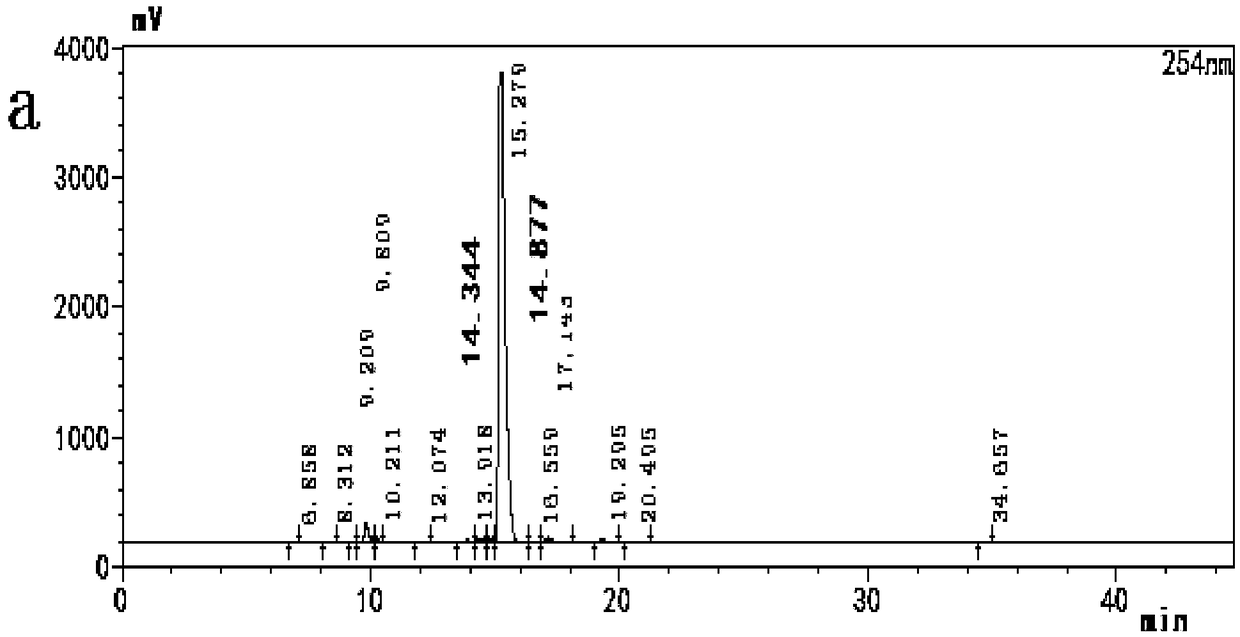
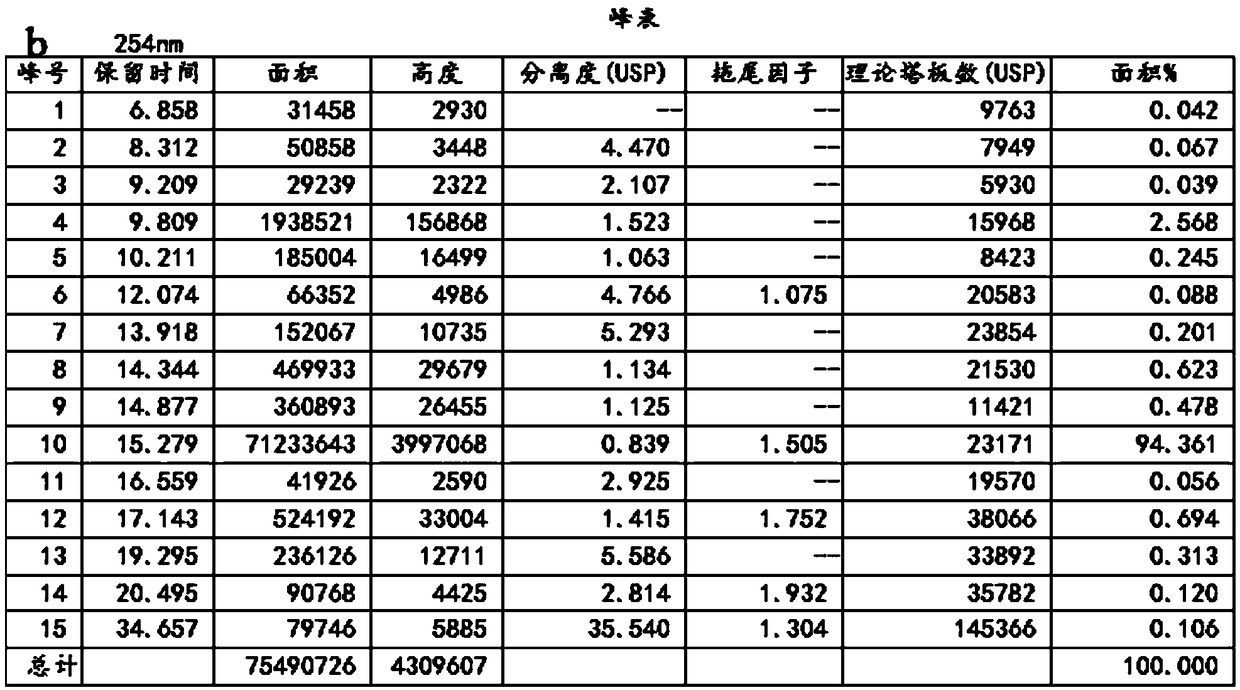
[0046] Add 3 g of potassium bromate, 30 g of potassium bromide, and 80 g of water into the three-necked flask, and stir to dissolve. Then add 90 g of ethyl acetate and 10 g of rifamycin S, continue stirring to dissolve completely, and lower the temperature to 10°C. Add 20 g of 30% sulfuric acid solution dropwise, drop it in 1-2 hours, and react at 40°C for 8 hours after dropping. They were washed with 2% sodium bicarbonate solution, 2% hydrochloric acid solution and saturated brine respectively, 150 g of n-hexane was added to the organic layer, the temperature was lowered to 10° C., and the crystallization was stirred for 3 h. Suction filtration, dry, obtain 3-bromorifamycin S10.2g, yield is 88.3%, HPLC content is 98.90%, the HPLC detection result of gained 3-bromorifamycin S is as shown in Figure 2, detection wavelength 254nm.
Embodiment 2
[0048] A kind of preparation of rifabutin intermediate 3-bromorifamycin S
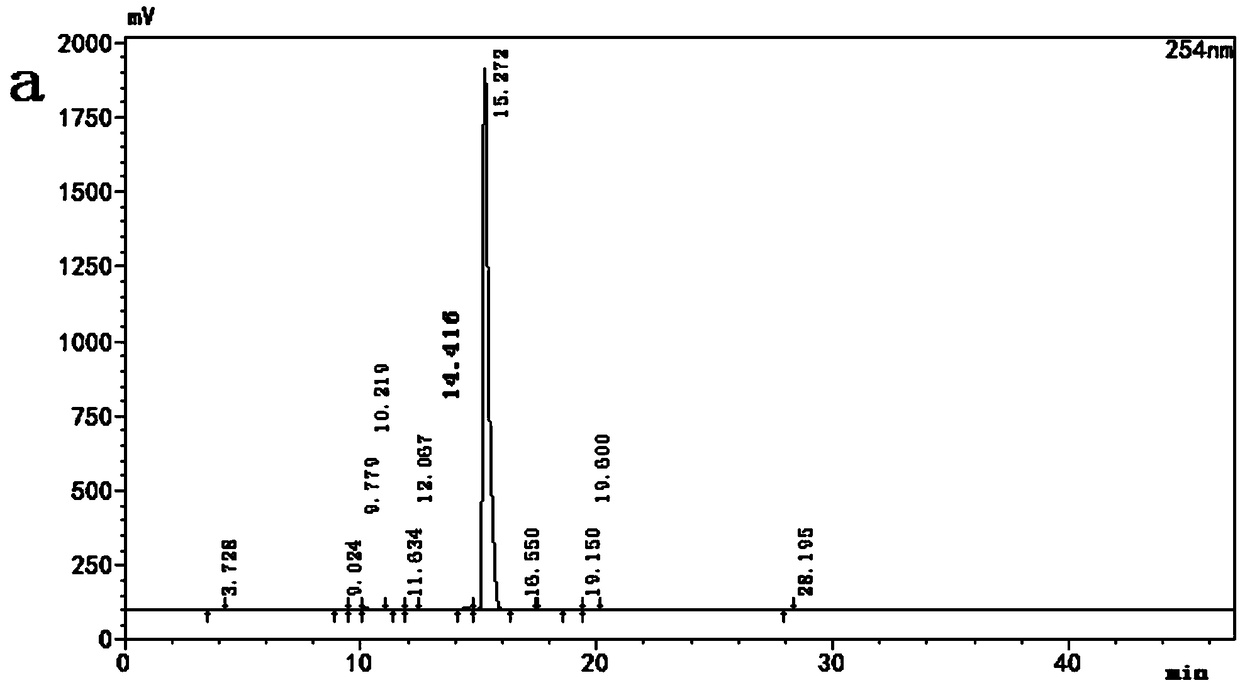
[0049] Add 3 g of potassium bromate, 15.0 g of potassium bromide, and 70 g of water into the three-necked flask, and stir to dissolve. Then add 90 g of ethyl acetate and 10 g of rifamycin S, continue stirring to dissolve completely, and lower the temperature to 5°C. 45g of 20% sulfuric acid solution was added dropwise, and the drop was completed in 1-2 hours, and reacted at about 25°C for 3 hours after the drop was completed. Wash with 2% sodium thiosulfate solution, 1% sulfuric acid solution and saturated brine respectively, add 150 g of n-heptane to the organic layer, lower the temperature to 7° C., stir and crystallize for 2.5 h. After suction filtration and drying, 9.8 g of 3-bromorifamycin S was obtained, the yield was 91.2%, and the HPLC content was 98.85%. The HPLC detection result of the obtained 3-bromorifamycin S is shown in FIG. 3 .
Embodiment 3
[0051] A kind of preparation of rifabutin intermediate 3-bromorifamycin S
[0052] Add 3 g of potassium bromate, 3 g of potassium bromide, and 80 g of water into the three-necked flask, and stir to dissolve. Then add 90 g of ethyl acetate and 10 g of rifamycin S, continue stirring to dissolve completely, and lower the temperature to 0°C. Add 50 g of 30% hydrochloric acid solution dropwise, drop it in 1-2 hours, and react at -5°C for 1 hour after dropping.
[0053] They were washed with 2% sodium carbonate solution, 1% hydrochloric acid solution and saturated brine respectively, 12 g of isopropyl ether was added to the organic layer, the temperature was lowered to -5°C, and the mixture was stirred and crystallized for 2 h. After suction filtration and drying, 10.0 g of 3-bromorifamycin S was obtained with a yield of 90.1% and an HPLC content of 98.25%. The HPLC detection results of the obtained 3-bromorifamycin S are shown in FIG. 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com