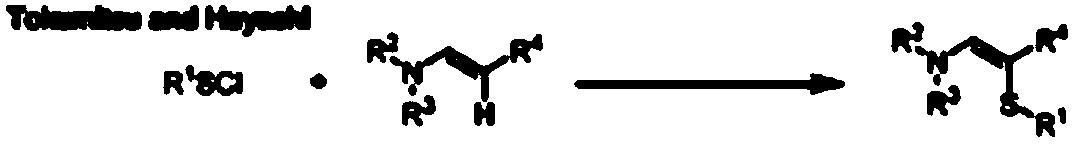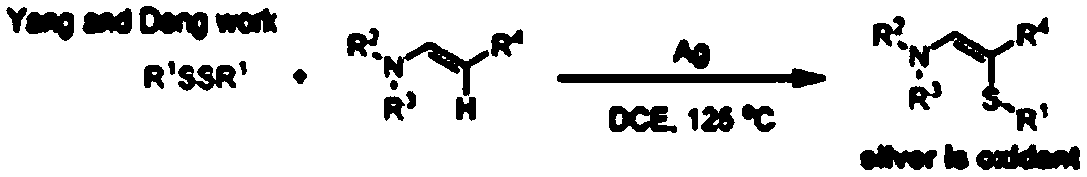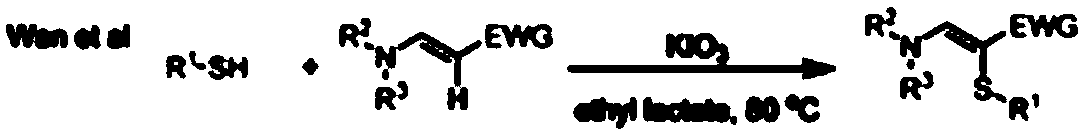Green synthesis method of alpha-thioenamine compound
A thioenamine, green synthesis technology, applied in the field of chemistry, can solve the problems of increased production cost, poor atom economy, a large number of chemical wastes, etc., to avoid waste and metal residues, promote the improvement of synthesis efficiency, and the synthesis process. Simple and efficient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Add enamine (45.0μL, 0.2mmol), electrolyte tetrabutyl ammonium tetrafluoroborate (32.9mg, 50mol%, that is, 50% of the amount of enamine compounds), p-chlorophenylsulfur Phenol (57.8mg, 0.4mmol) was inserted into the electrode. After pumping 3 times under a nitrogen atmosphere, 10mL of acetonitrile was added, and then electrolyzed at a constant current of I=3mA for 5h. After the reaction, the product was separated and purified by column chromatography. The rate is 89%. 1 H NMR (400MHz, CDCl 3 )δ10.15(t,J=6.1Hz,1H),7.39–7.28(m,3H),7.17–7.11(m,2H),7.08–7.03(m,2H),6.98–6.91(m,2H) ,5.80–5.70(m,1H),5.25–5.04(m,2H),4.18(q,J=7.1Hz,2H),3.58–3.54(m,2H),1.17(t,J=7.1Hz,3H ). 13 C NMR (101MHz, CDCl 3 )δ171.38,171.07,140.33,134.53,134.17,129.52,128.92,128.30,128.18,126.58,126.02,116.52,82.92,60.18,47.79,14.38. 20 h 20 ClNNaO 2 S[M+Na] + :396.0795; found: 396.0803.
Embodiment 2
[0053]
[0054] Add enamine (45.0μL, 0.2mmol), electrolyte tetrabutyl ammonium tetrafluoroborate (32.9mg, 50mol%, that is, 50% of the amount of enamine compounds), p-bromophenylsulfur Phenol (75.6mg, 0.4mmol) was inserted into the electrode. After pumping 3 times under a nitrogen atmosphere, 10mL of acetonitrile was added, and then electrolyzed at a constant current of I=3mA for 5h. After the reaction was completed, the product was separated and purified by column chromatography. Rate 80%. 1 H NMR (400MHz, CDCl 3 )δ10.15(d,J=5.7Hz,1H),7.45–7.21(m,5H),7.10–7.00(m,2H),6.95–6.79(m,2H),5.79–5.70(m,1H) ,5.26–5.05(m,2H),4.18(q,J=7.1Hz,2H),3.66–3.48(m,2H),1.17(t,J=7.1Hz,3H). 13 CNMR (101MHz, CDCl 3 )δ171.38, 171.02, 141.03, 134.49, 134.14, 131.15, 128.91, 128.17, 126.54, 126.30, 117.26, 116.51, 82.70, 60.17, 47.78, 14.37. HRMS (ESI) calculated for C 20 h 20 BrNNaO 2 S[M+Na] + :440.0290; found: 440.0293.
Embodiment 3
[0056]
[0057] Add enamine (45.0μL, 0.2mmol), electrolyte tetrabutylammonium tetrafluoroborate (32.9mg, 50mol%, that is, 50% of the amount of enamine compounds), p-fluorobenzenesulfur Phenol (51.3mg, 0.4mmol) was inserted into the electrode. After pumping 3 times under a nitrogen atmosphere, 10mL of acetonitrile was added, and then electrolyzed at a constant current of I=3mA for 5h. After the reaction was completed, the product was separated and purified by column chromatography. rate of 76%. 1 H NMR (400MHz, CDCl 3 )δ10.04(t,J=6.3Hz,1H),7.31–7.21(m,3H),7.03–6.95(m,2H),6.92–6.86(m,2H),6.84–6.77(m,2H) ,5.72–5.62(m,1H),5.18–5.00(m,2H),4.12(q,J=7.1Hz,2H),3.49–3.46(m,2H),1.10(t,J=7.1Hz,3H ). 19 F NMR (377MHz, CDCl 3 )δ-119.42. 13 C NMR (101MHz, CDCl 3 )δ171.20, 171.17, 160.47 (J C-F =242.7Hz), 136.53(J C-F =3.0Hz), 134.66, 134.24, 128.85, 128.13, 126.86, 126.77 (J C-F =2.7Hz), 116.46, 115.24 (J C-F =21.7Hz), 84.08, 60.13, 47.77, 14.37. HRMS (ESI) calculated for C 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com