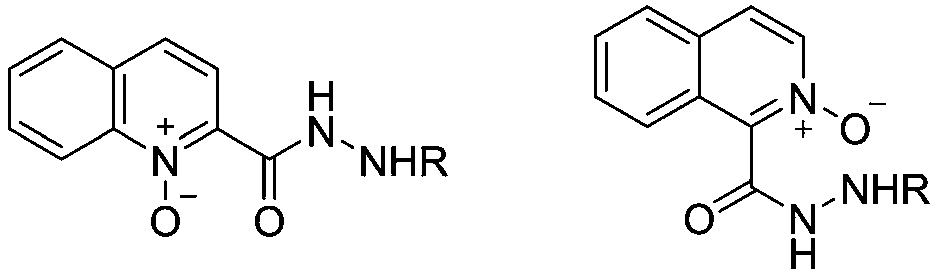
N-arylation method in aqueous phase system with substituted quinoline or isoquinoyl hydrazide pyridine-N-oxide as ligand
A quinoline hydrazide pyridine, arylation technology, applied in the chemical field, can solve problems such as complex ligand structure, cumbersome synthesis steps, and difficulty in obtaining it, and achieve the effects of broad application prospects, simple operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
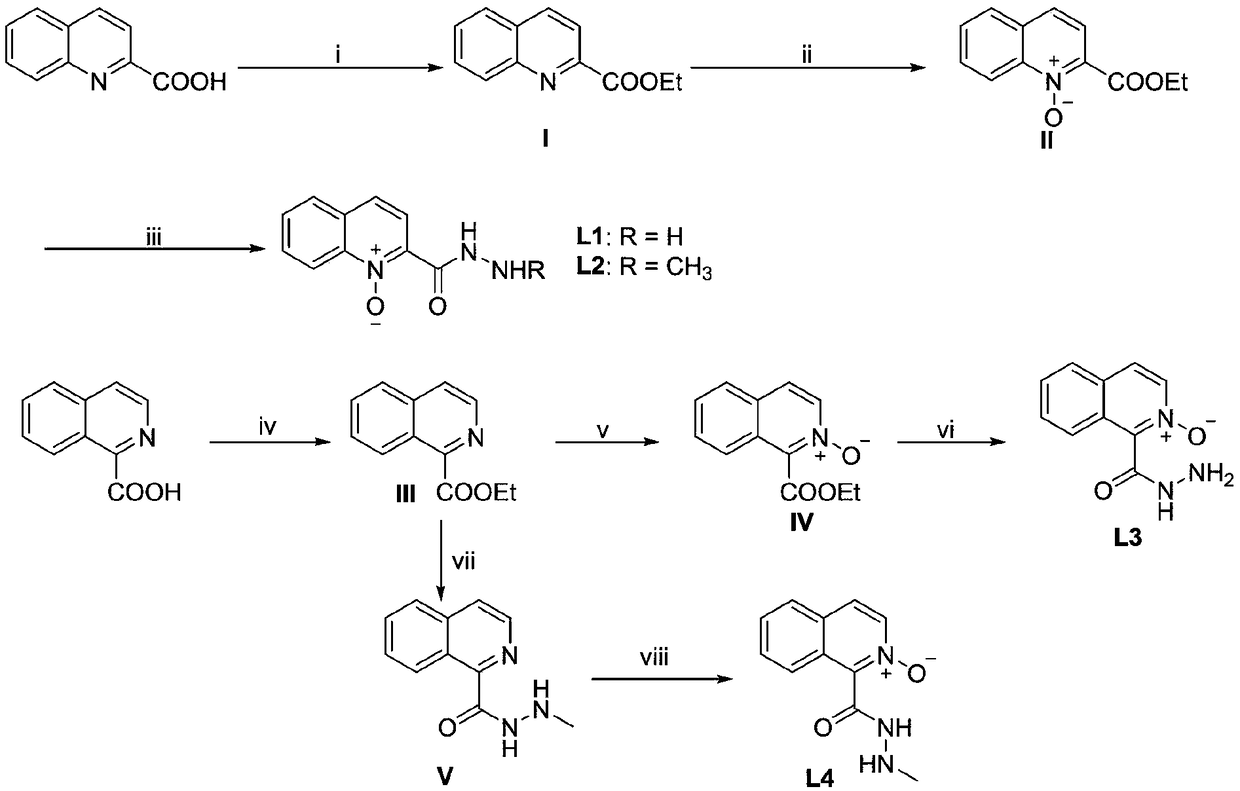
[0039] The preparation method of ligand in the present invention is as figure 1 As shown, the specific operation steps are as follows:
[0040] The process of reaction i is: take 2-quinoline formic acid (1.732g, 10mmol) in a 100mL round bottom flask, add 3mL of ethanol to dissolve, and add 6mL of thionyl chloride dropwise within 15 minutes under ice-water bath conditions. Subsequently, it was heated under reflux for 8 hours, and TLC detected that the reaction was complete. After the reaction was completed, the ethanol was distilled off, and saturated NaHCO was added 3 The solution was adjusted to pH 7. After extraction with ethyl acetate, anhydrous Na 2 SO 4 The organic layer was dried, and the solvent was spin-dried to obtain the crude product of compound I. The product was then separated by column chromatography (eluent: petroleum ether-ethyl acetate) to obtain compound I (1.80 g, 90%) as a colorless oil.
[0041] The process of reaction ii was as follows: compound I (...
Embodiment 1
[0050] Embodiment 1: the synthesis of N-p-methoxyphenylimidazole
[0051] Its reaction formula is as follows:
[0052]
[0053] 8mg (0.05mmol) Cu 2 O, 21.7mg (0.1mmol) ligand, 119.4mg (0.5mmol) p-methoxyiodobenzene, 51mg (0.75mmol) imidazole, 40mg (1.0mmol) NaOH, 32.3mg (0.1mol) TBAB, 1.0ml EtOH: h 2 O(v / v)=1:1 was added into a 10ml Schlenk reaction tube, stirred and reacted by heating with a heating plate, and reacted at 120°C for 12 hours. After the reaction stopped, add 10ml of water, extract with ethyl acetate (3×20ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and pass the obtained reaction mixture through a silica gel column Separation and purification by column chromatography [eluent: petroleum ether / ethyl acetate (2:1)] gave 84 mg of N-p-methoxyphenylimidazole with a yield of 96%.
[0054] N-p-methoxyphenylimidazole: 1 H NMR (400MHz, CDCl 3 ): δ7.80(s, 1H), ...
Embodiment 2
[0055] Embodiment 2: the synthesis of N-p-methoxyphenylpyrrole
[0056] Its reaction formula is as follows:
[0057]
[0058] 4.4mg (0.05mmol) CuO, 21.7mg (0.1mmol) ligand, 119.4mg (0.5mmol) p-methoxyiodobenzene, 100mg (1.5mmol) pyrrole, 40mg (1.0mmol) NaOH, 32.3mg (0.1mmol) )TBAB, 1.0ml EtOH:H 2 O(v / v)=1:1 was added into a 10ml Schlenk reaction tube, stirred and reacted by heating with a heating plate, and reacted at 100°C for 14 hours. After the reaction stopped, add 10ml of water, extract with ethyl acetate (3×20ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and pass the obtained reaction mixture through a silica gel column Separation and purification by column chromatography [eluent: petroleum ether / ethyl acetate (10:1)] gave 81 mg of N-p-methoxyphenylpyrrole with a yield of 93%.
[0059] N-p-methoxyphenylpyrrole: 1 H NMR (400MHz, CDCl 3 ): δ7.34–7.32(m, 1H), 7.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com