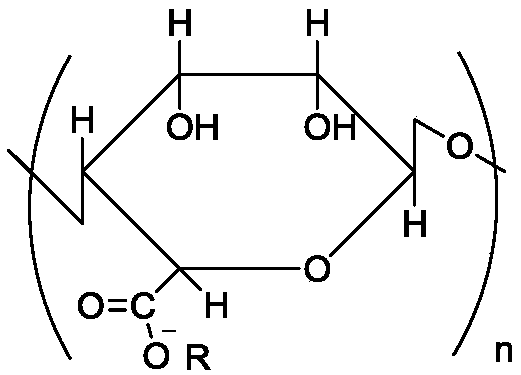
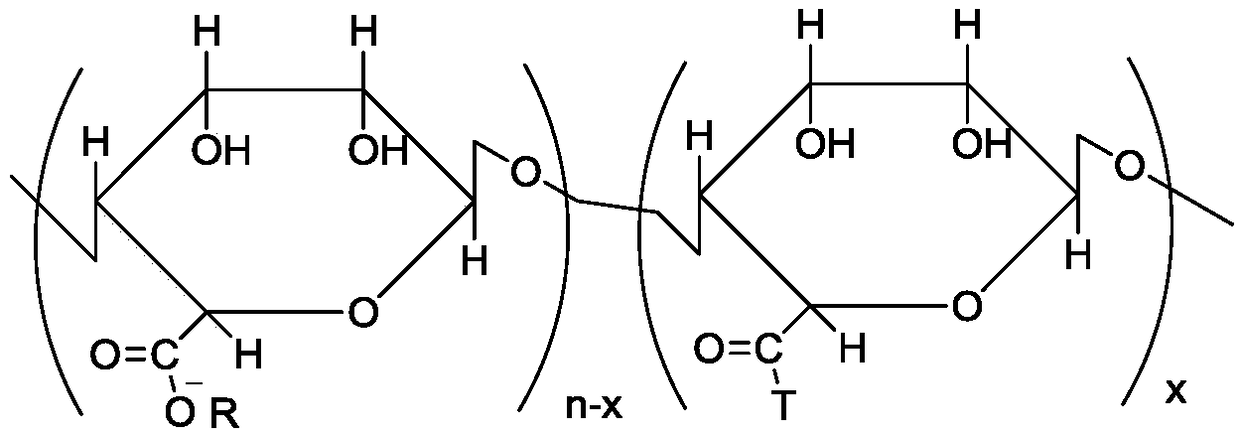
Preparation method and use of modified nitrogen-oxygen free radical polymerization inhibitor
A technology of nitroxide free radical and polymerization inhibitor, which is applied in the field of organic synthesis catalysis/inhibition, can solve the problems of affecting the polymerization inhibitory effect of the polymerization inhibitor and the difficulty of removing the high-efficiency polymerization inhibitor, and achieve the effect of convenient removal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Weigh 10g of sodium alginate with a molecular weight of 5000 and dissolve it in double distilled water, stir evenly, the system is in a completely dissolved state, slowly add HCl dropwise to the system, adjust the pH value to 4, and then pour the solution into ethanol : HCl=96:4 (v / v), the solid was collected by filtration, the obtained solid was washed with ethanol, and dried to obtain alginic acid solid.
[0059] (2) Take 5 g of alginic acid solid and disperse it in double distilled water, neutralize it with tetrabutylammonium hydroxide, finally adjust the pH value of the system to 6.5, and freeze-dry to obtain tetrabutyl alginate, an oil-soluble polysaccharide.
[0060] (3) Take 5g of tetrabutyl alginate, an oil-soluble polysaccharide, and dissolve it in 10ml of dimethylformamide solution, stir to dissolve it completely, then add 50mg of 2-chloro-1-methyliodopyridine, and place in an ice-water bath (0°C), catalyzed for 1 h under nitrogen. Then the temperature wa...
Embodiment 2
[0063] (1) Weigh 30g of sodium alginate with a molecular weight of 10,000 and dissolve it in double distilled water, stir evenly, the system is in a completely dissolved state, slowly add sulfuric acid to the system, adjust the pH value to 5, and then pour the solution into ethanol : HCl=90:10 (v / v), the solid was collected by filtration, the obtained solid was washed with methanol, and dried to obtain alginic acid solid.
[0064] (2) Take 20 g of alginic acid solid and disperse it in double distilled water, neutralize it with tetramethylammonium hydroxide, finally adjust the pH value of the system to 7.5, and freeze-dry to obtain tetramethyl alginate, an oil-soluble polysaccharide.
[0065] (3) Take 25g of tetramethyl alginate, an oil-soluble polysaccharide, and dissolve it in 25ml of diethylformamide solution, stir to dissolve it completely, then add 5g of 2-chloro-1-methyliodopyridine, and place in an ice-water bath (0°C), catalyzed for 1 h under nitrogen. Then the tempera...
Embodiment 3
[0073] (1) Weigh 50g of sodium alginate with a molecular weight of 12000 and dissolve it in double distilled water, stir evenly, the system is in a completely dissolved state, slowly add nitric acid dropwise to the system, adjust the pH to 4.5, and then pour the solution into ethanol : HCl=98:2 (v / v), the solid was collected by filtration, the obtained solid was washed with propanol, and dried to obtain alginic acid solid.
[0074] (2) Take 40 g of alginic acid solid and disperse it in double distilled water, neutralize it with tetraethylammonium hydroxide, finally adjust the pH value of the system to 7, and freeze-dry to obtain tetraethyl alginate, an oil-soluble polysaccharide.
[0075] (3) Take 30g of oil-soluble polysaccharide alginic acid tetramethyl salt, dissolve it in 30ml of diethylformamide solution, stir to make it dissolve completely, then add 2.1g of 2-chloro-1-methyl iodopyridine, in ice Water bath (0°C), catalyzed under nitrogen for 1h. Then the temperature was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com