Preparation and application of a kind of maltogenic amylase mutant
A maltogenic amylase and mutant technology, which is applied in the field of enzyme engineering, can solve the problems of reducing product purity and reducing the final yield of maltose, and achieves the effects of less conversion by-products, important industrial application potential, and high conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of wild maltogenic amylase.
[0021] (1) Construction of maltogenic amylase recombinant bacteria
[0022] According to the amyM amino acid sequence on NCBI (NCBI number: AAA22233.1), the sequence was codon-optimized, and the gene sequence amyM of maltogenic amylase was synthesized by chemical total synthesis. The plasmid used to construct the expression vector in E. coli is pET24a(+). The pET24a(+) plasmid and the plasmid with the amyM gene were subjected to NcoI and HindIII double enzyme digestion respectively. After the digestion products were recovered by gel, they were ligated with T4 ligase overnight, and the ligated products were transformed into Escherichia coli JM109 competent cells, and the transformed products Spread on LB plates containing 100mg / L kanamycin, culture overnight at 37°C, pick 2 single colonies on the plate, insert them into LB liquid medium, extract plasmids for verification after 8 hours, the result is correct, and enric...
Embodiment 2
[0026] Embodiment 2: preparation of maltogenic amylase mutant
[0027] (1) Single mutation
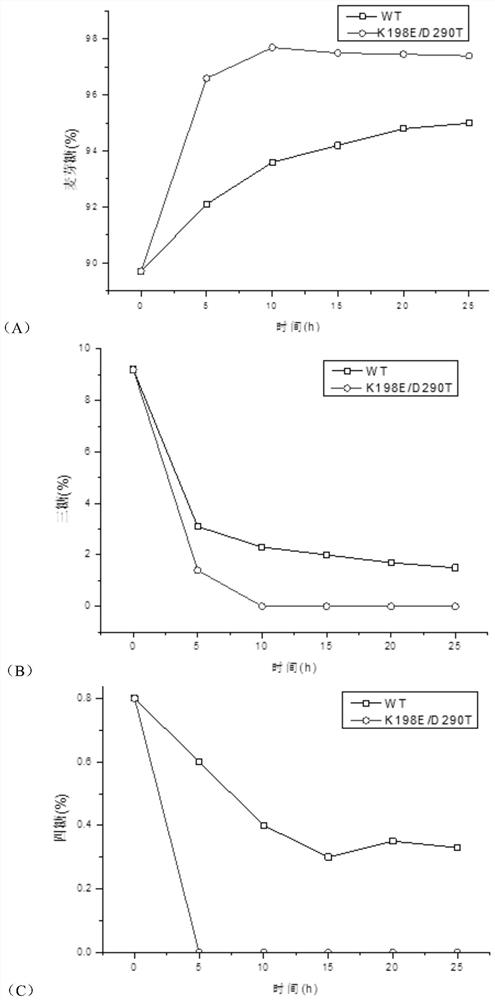
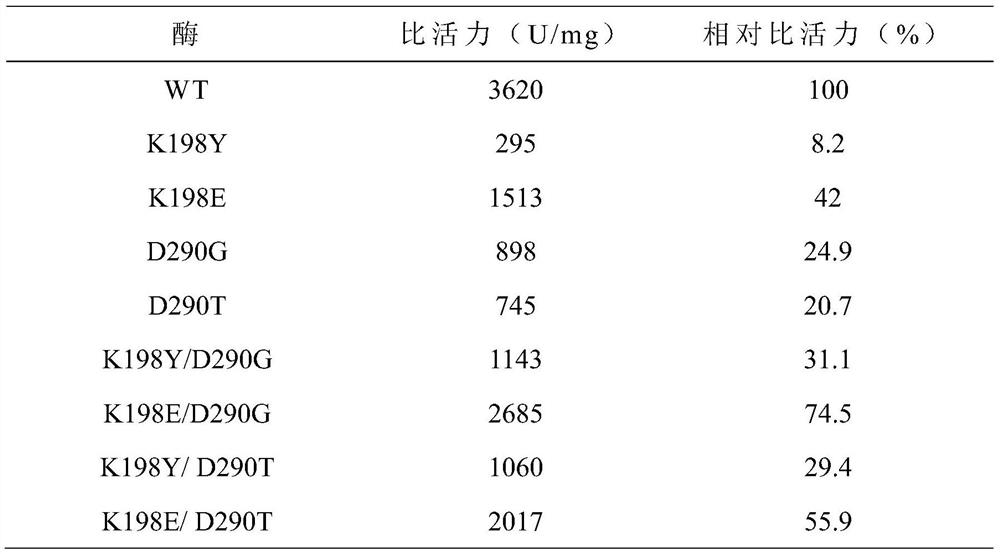
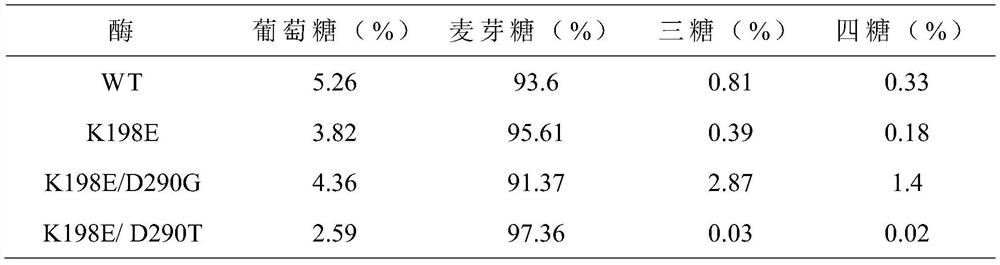
[0028] The 198th lysine (Lys) in the maltose amylase was mutated into glutamic acid (Glu) and tyrosine (Tyr) respectively, marked as K198E, K198Y; the 290th asparagus in the maltose amylase Amino acid (Asp) was mutated into glycine (G, ly) and threonine (Thr) respectively, labeled as D290G and D290T, respectively.
[0029] The site-directed mutagenesis primers for introducing the K198E mutation are:
[0030] Forward primer: 5'-CGACGACGCTACCGAAGGTTACTTCCACCA-3' (underlined is the mutated base)
[0031] Reverse primer: 5'-TGGTGGAAGTAACCTTCGGTAGCGTCGTCG-3' (the underline is the mutated base)
[0032] The site-directed mutagenesis primers for introducing the K198Y mutation are:
[0033] Forward primer: 5'-CGACGACGCTACCTATGGTTACTTCCACCA-3' (the underline is the mutated base)
[0034]Reverse primer: 5'-TGGTGGAAGTAACCATAGGTAGCGTCGTCG-3' (the underline is the mutated base)
[0035] The s...
Embodiment 3
[0046] Embodiment 3: Analysis of maltogenic amylase enzyme activity
[0047] (1) Definition of enzyme activity unit
[0048] When using the 3,5-dinitrosalicylic acid method (DNS method) to measure the activity of maltose amylase, the amount of enzyme needed to catalyze the production of 1 μmol of reducing sugar per minute is taken as an activity unit.
[0049] (2) Enzyme Activity Determination Steps
[0050] Preheating: Take 2mL of 0.5% soluble starch solution (50mM pH5.5 citric acid buffer) in a test tube and place it in a 60°C water bath to preheat for 10min.
[0051] Reaction: Add 0.1mL sample enzyme solution, oscillate evenly, accurately time 10min, add 3mL DNS, oscillate evenly, put into ice water to terminate the reaction, boil in a boiling water bath for 7min. cool down.
[0052] Measurement: add distilled water to the above reaction system and adjust the volume to 15mL, and mix well. The absorbance was measured at a wavelength of 540nm and the enzyme activity was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com