Fluorene photoinitiator and photocured composition comprising same
A photoinitiator and compound technology, applied in the field of photocuring, can solve the problem of easy migration of fluorene photoinitiators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
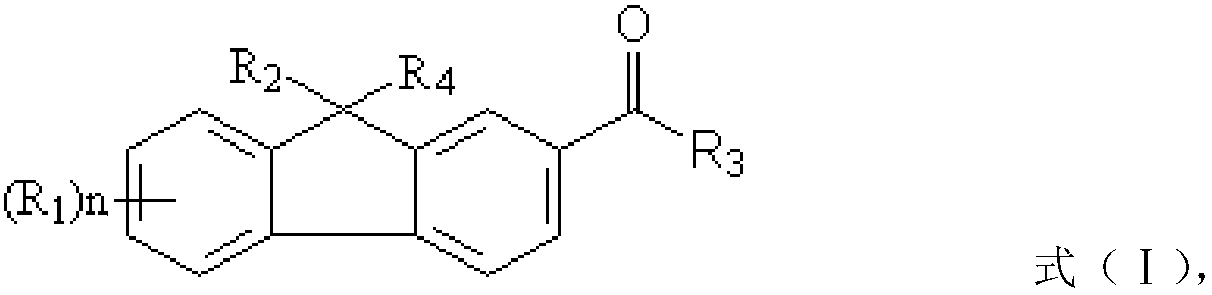
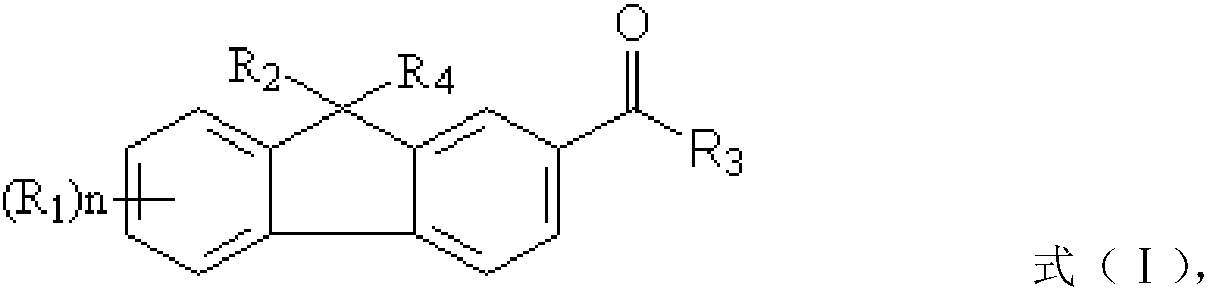
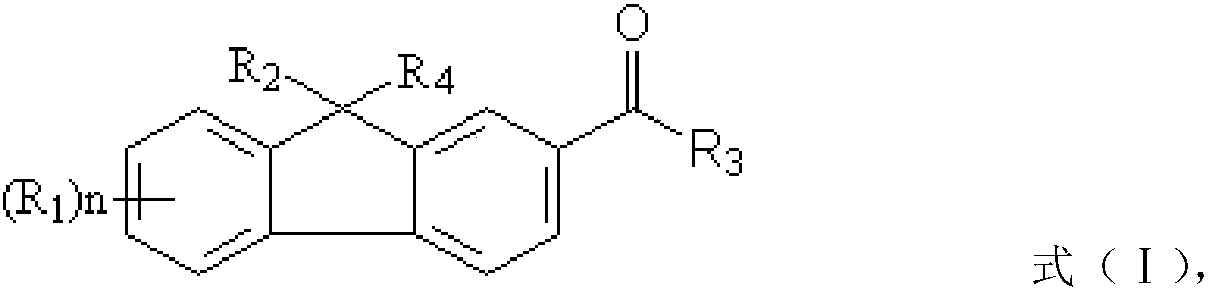
[0040] ① In compound a, R 2 and R 4 is hydrogen, compound b is a halogenated olefin or an acrylate containing a halogenated hydrocarbon; the preparation method also includes: under the action of a first catalyst, reacting compound a and compound b to obtain a fluorene photoinitiator; wherein, the first A catalyst is selected from one or more of the group consisting of sodium methoxide, sodium tert-butoxide, potassium tert-butoxide and potassium methoxide;
[0041] ② In compound a, R 2 and R 4 are independently selected from C 1 ~C 5 The carboxyl group substituted by the alkylene group, compound b is the alkene containing three-membered epoxy group and / or the (meth)acrylic acid ester containing three-membered epoxy group; The preparation method also includes: under the effect of the second catalyst, the Compound a and compound b undergo a chain extension reaction to obtain a fluorene photoinitiator, wherein the second catalyst is a basic catalyst of tertiary amines or quat...
Embodiment 1
[0070] 12.6 g of raw material 1a, 8.1 g of sodium methoxide, and 100 mL of thionyl chloride (DMSO) were added to a 250 mL four-necked flask to obtain a mixed solution. Nitrogen gas was introduced into the above-mentioned four-neck flask, and the above-mentioned mixture was stirred at room temperature for 0.5 h. Then, 11.4 g of the allyl chloride solution of the raw material 1b was slowly added dropwise to the above mixed solution, and the addition was completed within 3 hours, and the liquid phase followed the reaction until the raw material no longer changed. Then the reaction solution was poured into deionized water, stirred, the product was extracted with n-hexane, the n-hexane product solution was dried using anhydrous magnesium sulfate, then the n-hexane was removed by rotary evaporation, and finally recrystallized with methanol to obtain 13 g of a white solid product, namely Compound 1 has a purity of 98wt% and a yield of 78.2wt%. The synthetic route is as follows:
[...
Embodiment 2
[0076] 19.3 g of raw material 2a, 0.5 g of tetrabutylammonium bromide, and 50 mL of thionyl chloride (DMSO) were added to a 250 mL four-neck flask to obtain a mixed solution. Nitrogen gas was introduced into the above-mentioned four-neck flask, and the above-mentioned mixture was stirred at room temperature for 0.5 h. Then, a mixed solution of 12.8 g of raw material 2b and 50 mL of DMSO was slowly added dropwise to the above mixed solution, and the dropwise addition was completed within 3 hours, and the liquid phase followed the reaction until the raw material no longer changed. Then the reaction solution was poured into deionized water, stirred, the product was extracted with n-hexane, the n-hexane product solution was dried using anhydrous magnesium sulfate, then the n-hexane was removed by rotary evaporation, and finally recrystallized with methanol to obtain 26.3 g of a white solid product. Namely compound 2, the purity is 98wt%, and the yield is 82.8wt%. The synthetic ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com