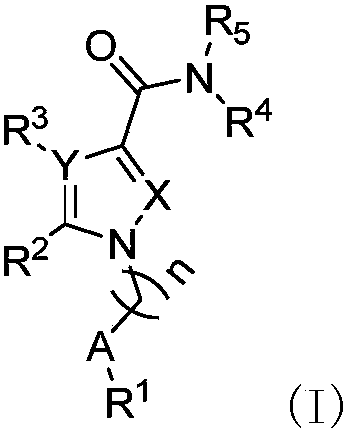
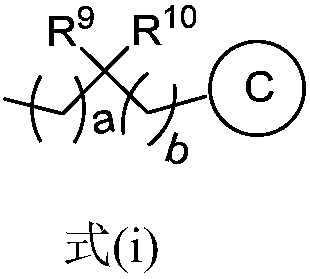
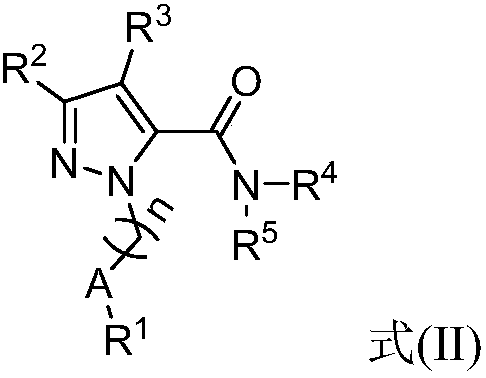
Nitrogen-containing five-membered heteroaromatic compound and preparation method and application thereof
A technology of aromatic heterocycles and compounds, which is applied in the field of nitrogen-containing five-membered aromatic heterocycles and their derivatives and their preparation, can solve the problem of lack of CB1 and CB2 selectivity, increase the risk of suicide for users, and limit cannabinoids. Problems such as drug development in the body, to achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0181]1 H-NMR was measured with a Bruker 500MHz instrument; MS was measured with a Bruker MicroTOF-Q LCMS instrument, and all were ESI methods unless specified; all solvents were re-distilled before use, and the anhydrous solvents used were all according to the standard method obtained by drying; except for the instructions, all reactions were carried out under the protection of argon and followed by TLC, and the post-treatment was washed with saturated brine and dried with anhydrous magnesium sulfate; the purification of the product was performed using silica gel (200 -300 mesh) column chromatography; the silica gel used, including 200-300 mesh and GF 254 Produced for Qingdao Ocean Chemical Factory or Yantai Yuanbo Silicone Company. Example 1-1, compound N-(2-(1-hydroxy-2-methyl)propyl)-3-tert-butyl-1-n-pentyl-1H-pyrazole-5-carboxamide (BE001) preparation of
[0182]
[0183] Take pinacolone (1002mg, 11.0mmol) in tetrahydrofuran (100ml), cool the reaction system with an ...
Embodiment 1-2 to 1-91
[0187] Preparation of BE series compounds shown in Examples 1-2 to 1-91 and Table 1 (see references below for the specific process)
[0188] Table 1
[0189]
[0190]
[0191]
[0192]
[0193]
[0194]
[0195]
[0196]
[0197]
[0198]
[0199]
[0200]
[0201]
Embodiment 1-92
[0202] Example 1-92, compound N-(2-(1-hydroxy-2-methyl)propyl)-5-tert-butyl-1-(4-fluorophenyl)-1H-pyrazole-3-methan Amide (BE092)
[0203]
[0204] Take pinacolone (200mg, 2.2mmol) in tetrahydrofuran (15ml), cool the reaction system with an ice-water bath, add potassium tert-butoxide (292mg, 2.9mmol), react at room temperature for 10min, and then add diethyl oxalate Ester (292mg, 2.4mmol), react overnight at room temperature. Continue to reflux 4-fluorophenylhydrazine hydrochloride (393mg, 2.4mmol) at 70°C for 3h in the reaction system. After the reaction was cooled to room temperature, the solvent was evaporated to dryness, extracted with ethyl acetate and water respectively,
[0205] After the organic phase was evaporated to dryness, the intermediate compound 5-tert-butyl-1-(4-fluorophenyl)-pyrazole-3-carboxylic acid ethyl ester (435 mg, 1.5 mmol) was obtained by column chromatography.
[0206] Dissolve ethyl 5-tert-butyl-1-(4-fluorophenyl)-pyrazole-3-carboxylate (435m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com