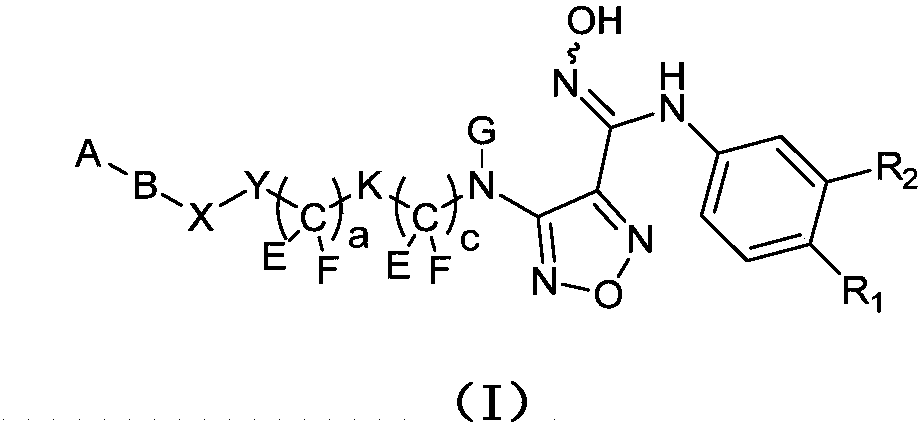
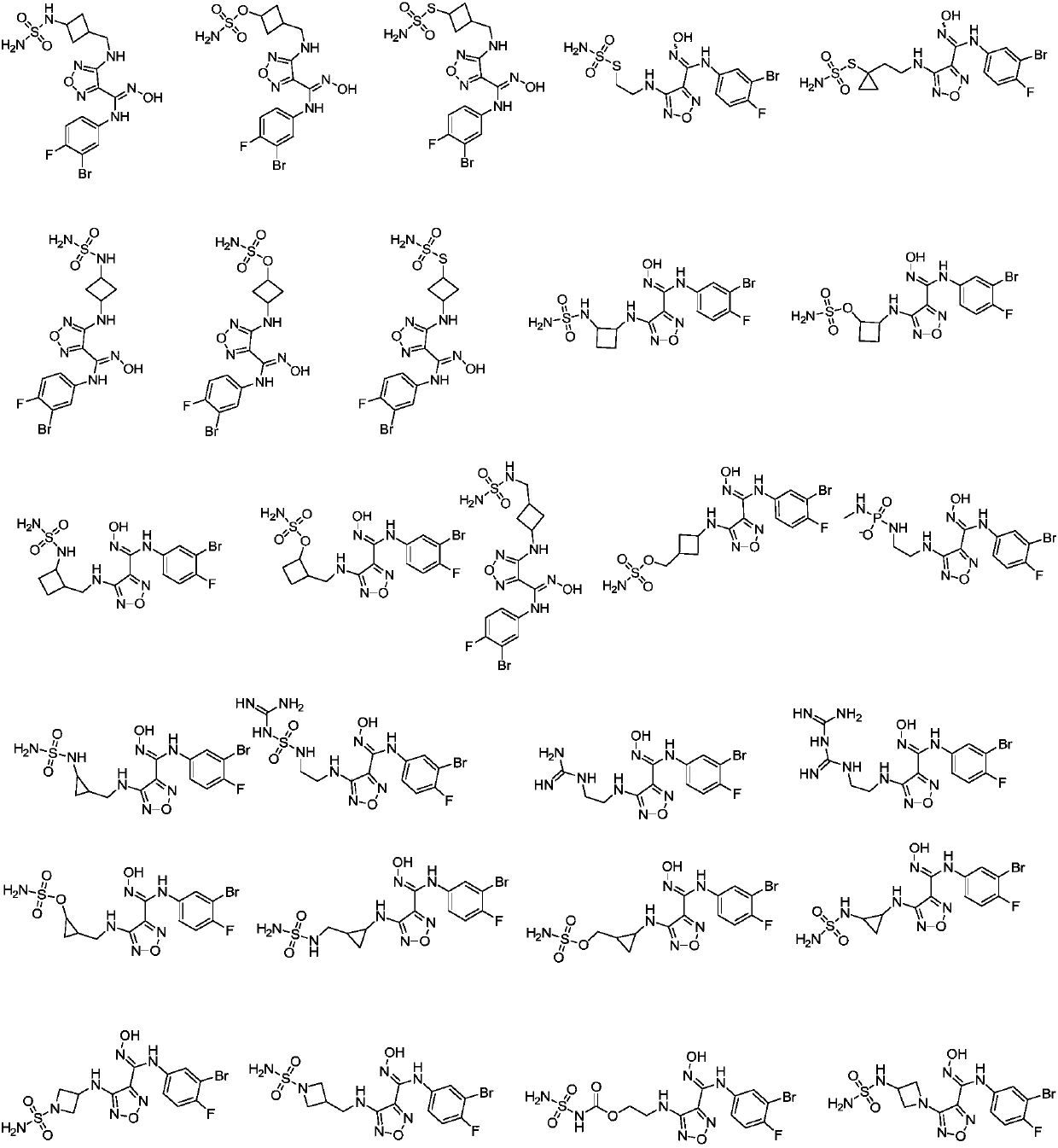
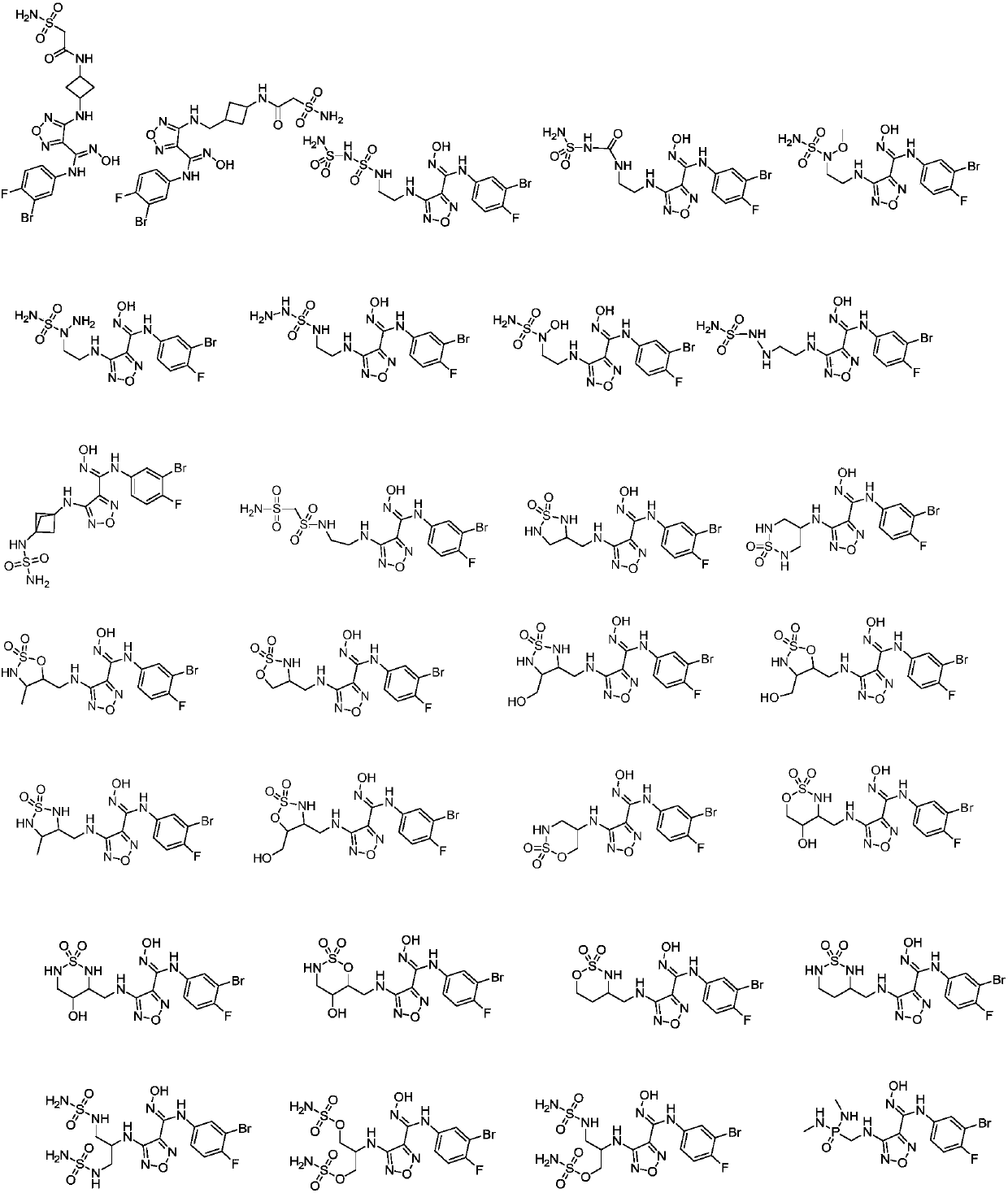
1,2,5-oxadiazole derivative and purpose thereof
A compound and mixture technology, applied in the field of medicine, can solve problems such as T-cell activation signal transduction blockage, achieve good therapeutic effects, broad application prospects, and significant therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of intermediate 7: 4-(3-bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazole-3-)-1,2,4- Oxadiazol-5-one
[0037]
[0038] step 1:
[0039]
[0040]Compound 1 (32g, 485mmol) was dissolved in 500mL of water and heated at 45°C until completely dissolved. Under cooling in an ice-water bath, sodium nitrite (38 g, 533 mmol) and 6N hydrochloric acid (5.5 mL) were added. After reacting in an ice bath for 0.5 hours, the temperature was raised to room temperature and reacted for 1.5 hours. The reaction solution was continued to be cooled in an ice bath, and 50% hydroxylamine hydrochloride aqueous solution (99 g, 1500 mmol) was added dropwise to the reaction solution, stirred for half an hour, and then raised to room temperature for 1 hour of reaction. Heat to reflux for 2 hours. After the reaction, adjust the pH to 7.0 with 80 mL of 6N hydrochloric acid in an ice bath. The precipitate was filtered, washed once with water, and dried to obtain compound 2 ...
Embodiment 2
[0054] Example 2 Synthesis of compound A: (E)-N-(3-bromo-4-fluorophenyl)-N′-hydroxyl-4-(3-sulfonylaminocyclobutylmethylamino)-1,2,5 -Oxadiazole-3-guanidine
[0055]
[0056] step 1:
[0057]
[0058] Dissolve compound 7 (150mg, 0.4mmol) and compound 8 (96mg, 0.6mmol) in 2mL of tetrahydrofuran, add dropwise triethylamine (202mg, 2.0mmol) at room temperature for 5 minutes, add water (10mL), ethyl acetate Extracted twice, dried over anhydrous sodium sulfate, concentrated, petroleum ether to ethyl acetate (8:1) column to give white solid compound 9 (190 mg, 97%).
[0059] Step 2:
[0060]
[0061] Compound 9 (190 mg, 0.40 mmol) was dissolved in 2 mL of dioxane, and dioxane hydrochloride (3 mL) was added to react at room temperature for 1 hour. After the reaction, the reaction solution was spin-dried to obtain white solid compound 10 (163 mg, 98%).
[0062] Step 3:
[0063]
[0064] Dissolve compound 10 (160mg, 0.35mmol) in anhydrous dichloromethane, add triethylam...
Embodiment 3
[0069] Example 3 Synthesis of compound B: (E)-N-(3-bromo-4-fluorophenyl)-N'-hydroxyl-4-(3-sulfonic acid cyclobutylmethylamino)-1,2,5 -Oxadiazole-3-guanidine
[0070]
[0071] step 1:
[0072]
[0073] Dissolve compound 7 (150mg, 0.4mmol) and compound 12 (85mg, 0.6mmol) in 2mL of tetrahydrofuran, add dropwise triethylamine (202mg, 2.0mmol) at room temperature for 5 minutes, add water (10mL), ethyl acetate Extracted twice, dried over anhydrous sodium sulfate, concentrated, petroleum ether to ethyl acetate (8:1) column to give white solid compound 13 (180 mg, 87%).
[0074] Step 2:
[0075]
[0076] Dissolve compound 13 (180mg, 0.35mmol) in anhydrous dichloromethane, add triethylamine (80mg, 0.7mmol), stir in an ice bath for a few minutes, add sulfamoyl chloride (40mg, 0.35mmol) dropwise, continue Stir for half an hour. After the reaction, the reaction solution was spin-dried to obtain white solid compound 14 (120 mg, 72%).
[0077] Step 3:
[0078]
[0079] Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com