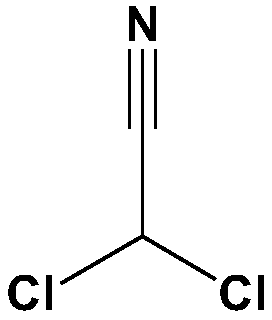
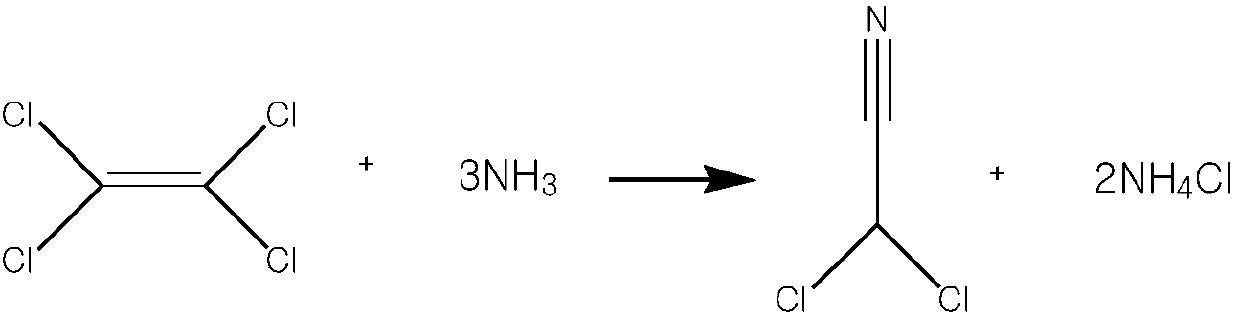
Method for synthesizing dichloroacetonitrile
A technology of dichloroacetonitrile and synthesis method, which is applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc., can solve problems such as high pressure, high environmental protection pressure, generation of bromide, etc., and achieve simple production process flow , Avoid the generation of three wastes, improve the yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 16.60g (0.1mol) of tetrachlorethylene and add it into a 100ml autoclave, then measure 33.20ml of dichloromethane into the autoclave and stir evenly, then raise the temperature to 60°C in a water bath, and slowly feed ammonia gas to 2.30°C while stirring. Mpa, heat preservation and pressure stirring for 4.0h, slowly cool down to 25°C and drain the remaining ammonia, then transfer the reaction solution to a 250ml four-necked bottle for distillation to recover dichloroacetonitrile, collect fractions at 110-120°C, and obtain dichloroacetonitrile The weight of acetonitrile is 9.63g, the purity of dichloroacetonitrile is 99.82%, and the yield is 87.51%.
Embodiment 2
[0026] Take 16.60g (0.1mol) of tetrachlorethylene and add it into a 100ml autoclave, then measure 33.20ml of dichloromethane into the autoclave and stir evenly, then raise the temperature to 60°C in a water bath, and slowly feed ammonia gas to 2.50°C while stirring. Mpa, heat preservation and pressure stirring for 4.0h, slowly cool down to 25°C and drain the remaining ammonia, then transfer the reaction solution to a 250ml four-necked bottle for distillation to recover dichloroacetonitrile, collect fractions at 110-120°C, and obtain dichloroacetonitrile The weight of acetonitrile was 10.19g, the purity of dichloroacetonitrile was 99.88%, and the yield was 92.61%.
Embodiment 3
[0028] Take 16.60g (0.1mol) of tetrachlorethylene and add it into a 100ml autoclave, then measure 33.20ml of dichloromethane into the autoclave and stir evenly, then raise the temperature to 60°C in a water bath, and slowly feed ammonia gas to 2.70°C while stirring. Mpa, heat preservation and pressure stirring for 4.0h, slowly cool down to 25°C and drain the remaining ammonia, then transfer the reaction solution to a 250ml four-necked bottle for distillation to recover dichloroacetonitrile, collect fractions at 110-120°C, and obtain dichloroacetonitrile The weight of acetonitrile was 10.10 g, the purity of dichloroacetonitrile was 99.85%, and the yield was 91.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com