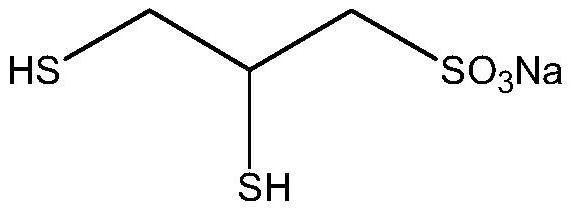
A kind of method for preparing 2,3-sodium dimercaptopropanesulfonate
A technology of sodium dimercaptopropanesulfonate and lead dimercaptopropanesulfonate, applied in the field of drug synthesis, can solve the problems of undisclosed yield and purity level, cumbersome operation process, shortened reaction time, etc., and achieve improved yield and purity , reduce the discharge of three wastes, and improve the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
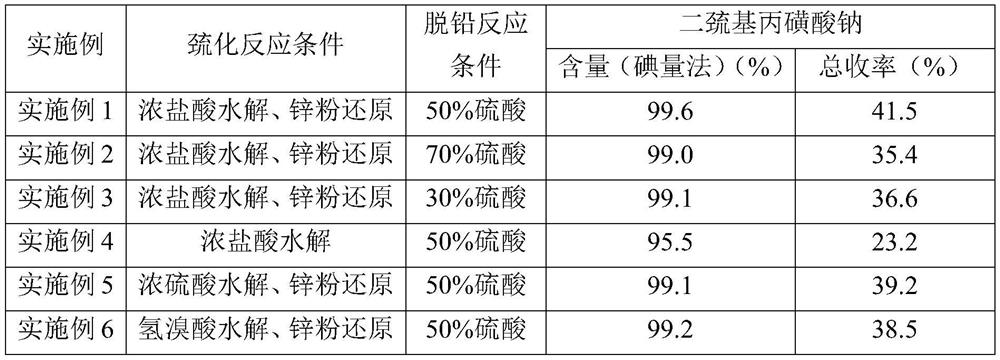
Examples
Embodiment 1
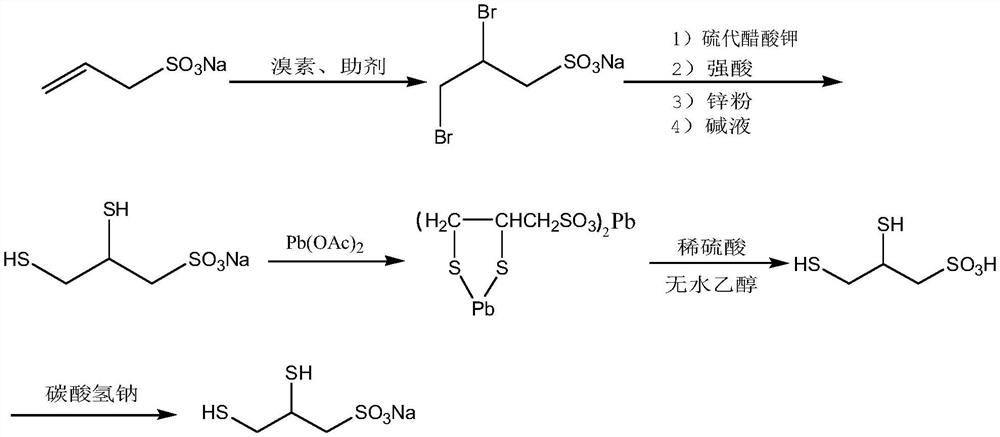
[0040] Concrete synthetic steps are as follows:
[0041] 1. Bromination reaction
[0042] Dissolve 7.5Kg of 95% sodium allyl sulfonate (49mol) in 30Kg of pure water, add 1Kg of sodium bromide (9.72mol) and stir to dissolve at room temperature, add 7.9Kg of bromine (49mol) dropwise, and the dropwise addition is completed in about 2 hours. Continue the reaction at room temperature for 1 h, stop the reaction, add a small amount of sodium sulfite until the solution becomes colorless, adjust the pH to 6-7 with 20% sodium carbonate, and obtain about 40 L of an aqueous solution of sodium 2,3-dibromopropanesulfonate.
[0043] 2. Thiolation reaction
[0044]Add 11.4Kg potassium thioacetate (98mol) to the above bromination solution at room temperature, heat up to 70-80°C, protect with nitrogen, keep warm for 2 hours, add 15L concentrated hydrochloric acid (36%-38%), keep warm for 3 hours , add 500g of zinc powder, keep warm for 4 hours, cool to room temperature, filter, adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com