Method for preparing lithium hydroxide by industrial-grade soluble lithium salt
A lithium hydroxide, soluble technology, applied in the direction of lithium oxide;/hydroxide, etc., can solve the problems of inability to obtain battery-grade lithium hydroxide, high production cost, and generation of calcium carbonate solid waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
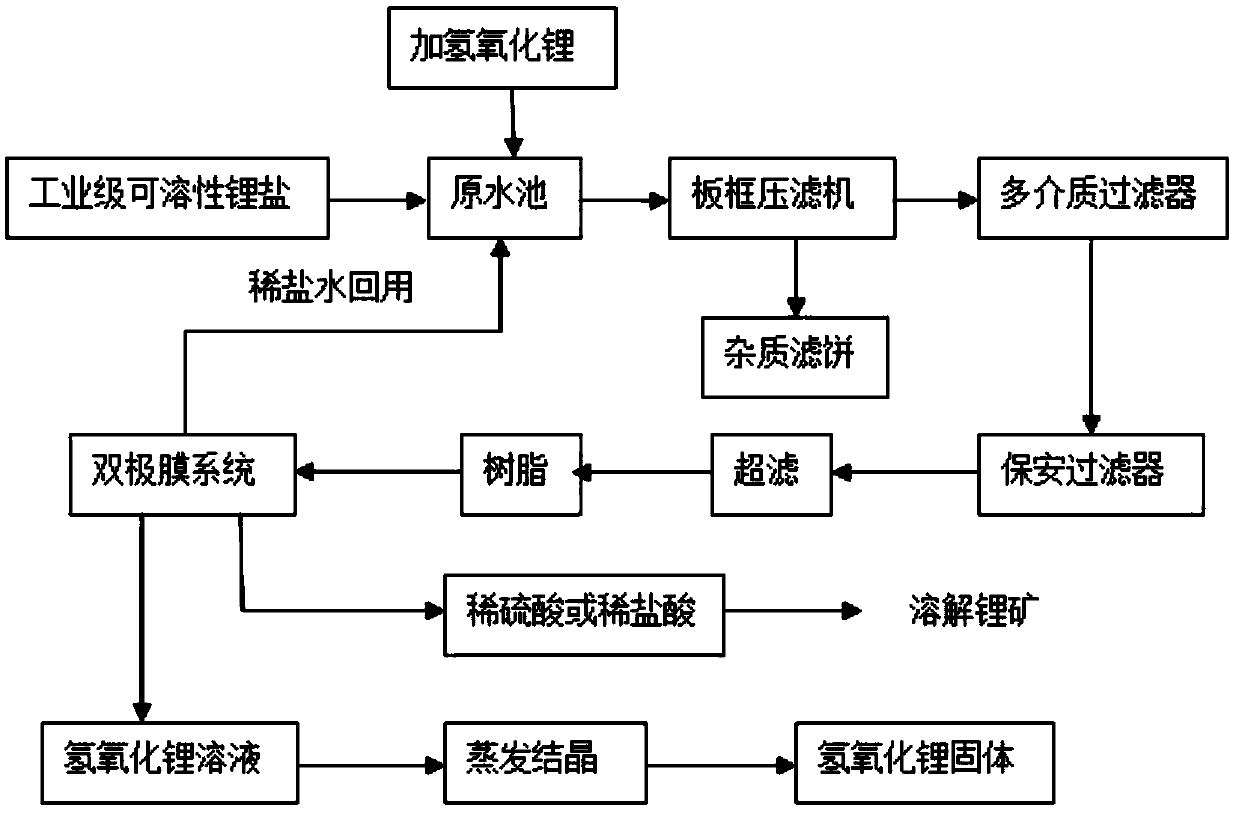
[0045]The industrial-grade lithium chloride solution is successively adjusted to pH 13 by lithium hydroxide, plate and frame filtration (the pressure of plate and frame filtration is 1.6 MPa, and the aperture of the filter frame is 5 microns), multi-media filtration (activated carbon, quartz sand and porous ceramics), ultrafiltration (pore size is 0.02 micron) and chelating resin adsorption, to obtain the pre-electrolyte;
[0046] The pre-electrolyte is carried out by bipolar membrane electrolysis (bipolar membrane cation exchange layer (N-type membrane), interfacial hydrophilic layer (catalyst layer) and anion-exchange layer (P-type membrane) are compounded and made, and the voltage of electrolysis is every pair The membrane is 1.5V, and the current density of electrolysis is 300A / m 2 , Lithium hydroxide was obtained by evaporation and crystallization once.
Embodiment 2
[0048] The industrial-grade lithium sulfate solution is successively adjusted to pH 13 by lithium hydroxide, plate and frame filtration (the pressure of the plate and frame filtration is 3 MPa, and the aperture of the filter frame is 8 microns), multi-media filtration (activated carbon, quartz sand and porous ceramics etc.), after ultrafiltration (pore size is 0.02 micron) and chelating resin adsorption, obtain pre-electrolyte;
[0049] The pre-electrolyte is carried out by bipolar membrane electrolysis (bipolar membrane cation exchange layer (N-type membrane), interfacial hydrophilic layer (catalyst layer) and anion-exchange layer (P-type membrane) are compounded and made, and the voltage of electrolysis is every pair The membrane is 2V, the current density of the electrolysis is 500A / m 2 , Lithium hydroxide was obtained by evaporation and crystallization once.
Embodiment 3
[0051] Adjust the pH value of industrial-grade lithium sulfate to 13 through lithium hydroxide, plate and frame filtration (the pressure of plate and frame filtration is 0.6 MPa, and the pore size of the filter frame is 10 microns), multimedia filtration (activated carbon, quartz sand, porous ceramics, etc.) etc.), after ultrafiltration (pore size is 0.02 micron) and chelating resin adsorption, obtain pre-electrolyte;
[0052] The pre-electrolyte is carried out by bipolar membrane electrolysis (bipolar membrane cation exchange layer (N-type membrane), interfacial hydrophilic layer (catalyst layer) and anion-exchange layer (P-type membrane) are combined to make, and the voltage of electrolysis is every The membrane is 2.5V, the current density of the electrolysis is 800A / m 2 , Lithium hydroxide was obtained by evaporation and crystallization once.
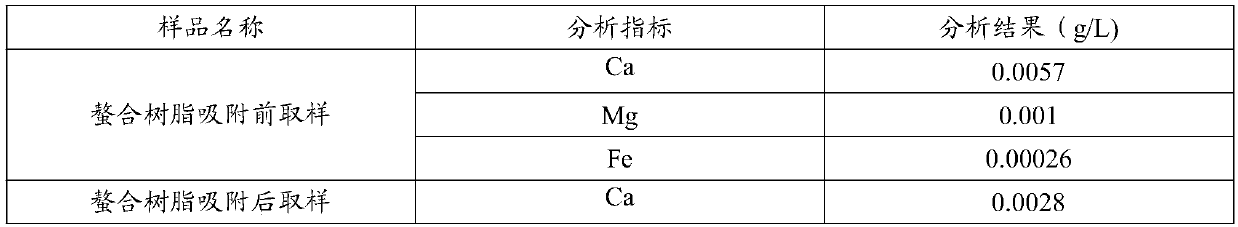
[0053] The liquid before and after the adsorption of embodiments 1-2 chelating resin is analyzed, and the results are shown in ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com