Jelly peptide special medical use formula food
A formula food, a special technology, applied in protein food processing, protein food ingredients, applications, etc., can solve the problems of special medical use and formula food for patients without hyperlipidemia, and achieve rich nutritional value, high-efficiency absorbability, and rapid absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of component
[0026] 1. Preparation of jellyfish hypolipidemic peptide by enzymatic method
[0027] (1) Processing of raw materials Weigh 50 kg of fresh jellyfish as raw material, soak in clear water, rehydrate, desalinate until the salt content is 4.5%, after cleaning, use a multifunctional pulverizer to pulverize, and the particle size of the jellyfish paste obtained is controlled at 120 μm;
[0028] (2) The first step of enzymatic hydrolysis to measure the water and protein content of the jellyfish pulp obtained in step (1), according to the measurement results, add water to adjust the protein mass concentration to 4%, adjust the pH value to 7.21, control the temperature to 52 ° C, add papaya The amount of protease and papain is to add 100u of papain per gram of protein, control the ratio of solid to liquid to 1.5g / ml, and heat-preserve for 8 hours to obtain the jellyfish papain hydrolyzate;
[0029] (3) The second step of enzymatic hy...
Embodiment 3
[0051] Embodiment 3: Preparation of formula food for special medical purpose
[0052] (1) Weigh each component by the following weight (g) (before packaging all raw material components, carry out quality inspection respectively to ensure that each component reaches the quality standard):
[0053] Soy protein isolate: 8g;
[0054] Whey protein: 5.3g;
[0055] Jellyfish peptide: 0.7g;
[0056] Vegetable fat powder: 32g;
[0057] Maltodextrin: 31g;
[0058] Isomalt: 13g;
[0059] Fructose-oligosaccharide: 2g;
[0060] Inulin: 2g;
[0061] (2) Put the above-mentioned raw materials into an automatic mixer and mix them for 15 minutes to obtain a uniform mixture;
[0062] (3) Put the homogeneous mixture into a fluidized bed for granulation. The granulation conditions are: use ethanol aqueous solution with a volume ratio of 65%, the material temperature is 45°C, the inlet air temperature is 90°C, the wind speed is 700rpm, and the peristaltic pump speed is 6.8 g / min;
[0063] ...
Embodiment 4
[0081] Example 4: Effect detection of formula food for special medical purpose
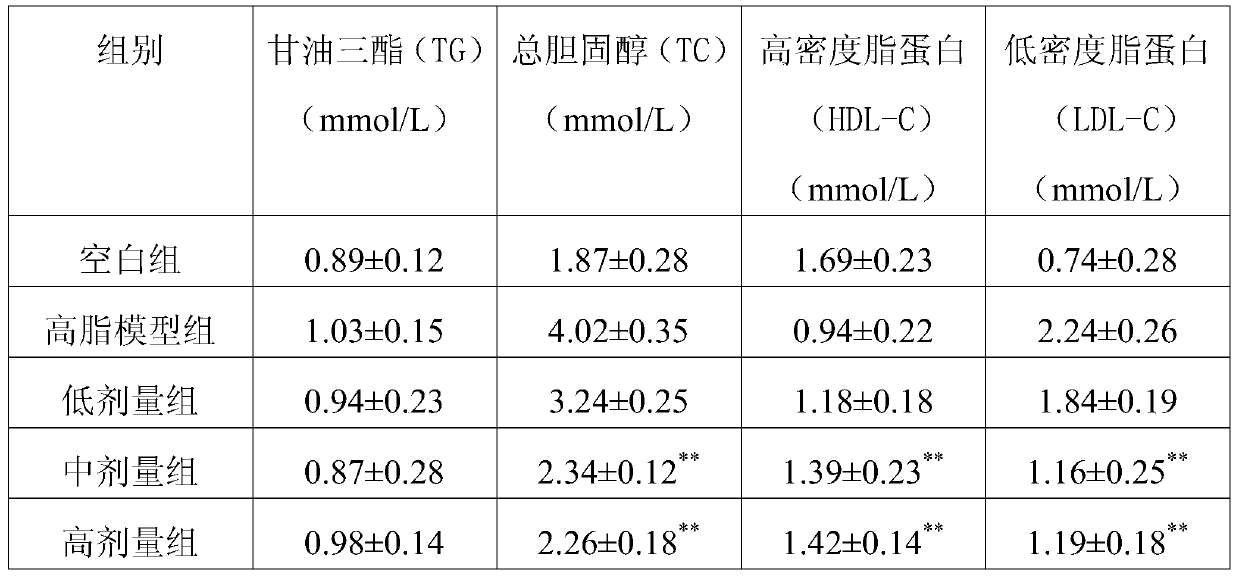
[0082] The product was tested according to the requirements of "General Rules for Formula Foods for Special Medical Purposes" (GB29922-2013), and the test results are as follows:
[0083] Table 2: Nutritional components of the formula food of the embodiment of the present invention
[0084]
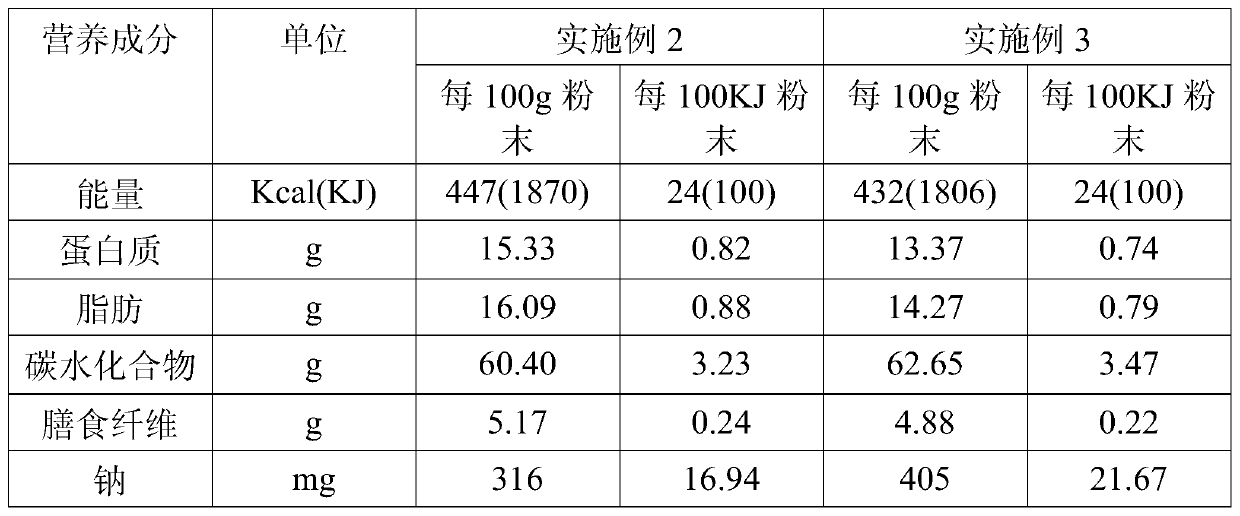
[0085] It can be seen from the test results in Table 2 that the formula food obtained by the present invention is comprehensive in nutrition, wherein the protein energy supply ratio of Example 3 accounts for 13.69%, the fat energy supply ratio accounts for 32.37%, and the carbohydrate energy supply ratio accounts for 53.94%. In Example 4, the protein energy supply ratio accounts for 12.36%, the fat energy supply ratio accounts for 29.71%, and the carbohydrate energy supply ratio accounts for 57.93%.
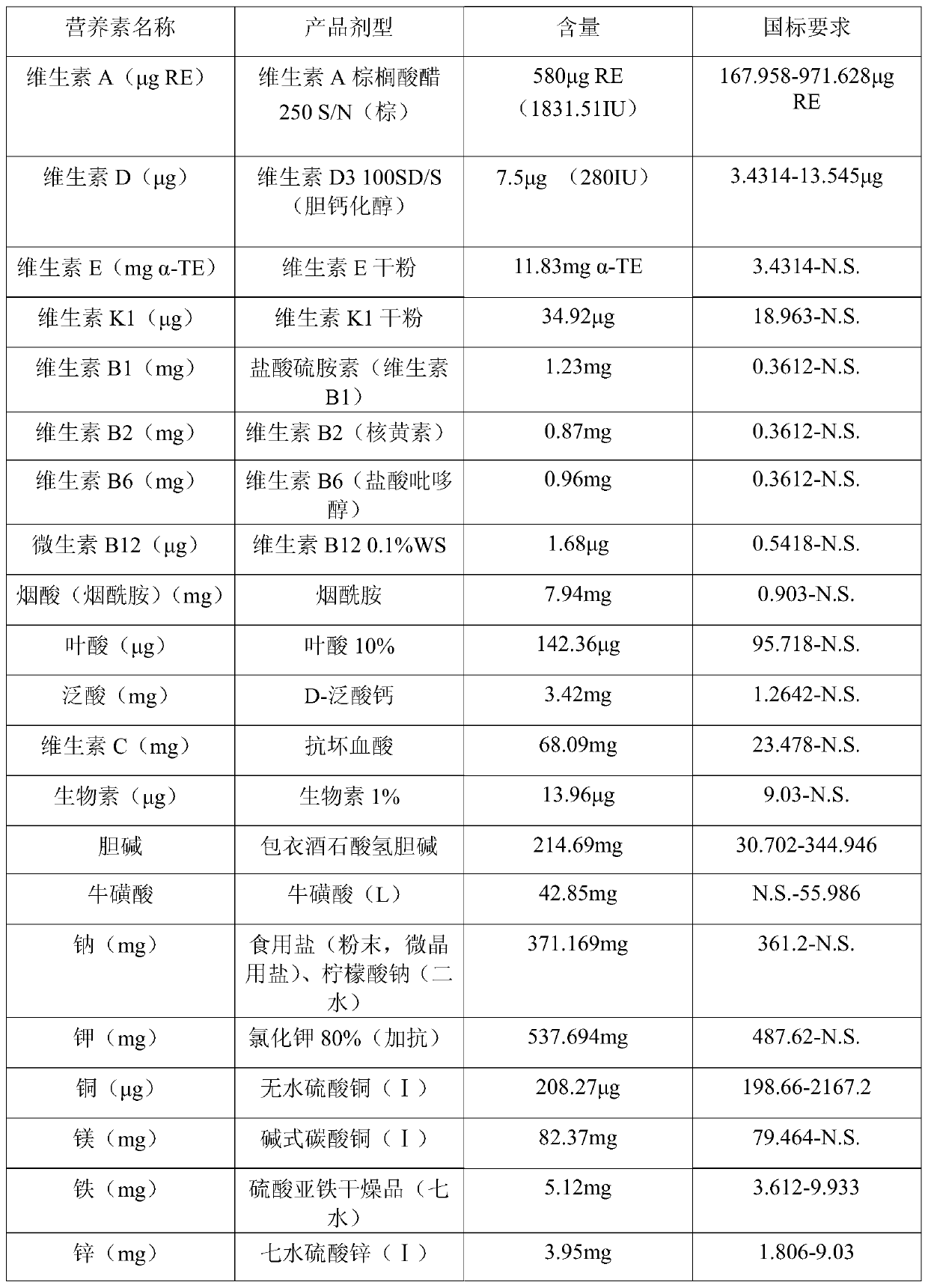
[0086] Table 3: Types and contents of vitamins and minerals in the formula food of Example 2 of the prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com