All-solid-state lithium ion electrolyte material and preparation method thereof
An electrolyte material, lithium ion technology, applied in chemical instruments and methods, circuits, electrical components, etc., can solve the limited improvement of grain boundary conductivity and total conductivity, the conductivity of electrolyte materials needs to be improved, and molten salt is mixed into powder. Body and other problems, to achieve the effect of excellent electrochemical performance, low cost, and improved conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Using lithium nitrate, aluminum nitrate, n-butyl titanate and phosphoric acid as raw materials, according to Li 1+x al x Ti 2-x (PO 4 ) 3 (Where x = 0.1, 0.2, 0.3, 0.4, 1, 1.1) stoichiometric ratio in the aqueous solution of ethanol, then add acetylacetone (according to 2 times the theoretical amount added), hydrolysis 8h under stirring at 60 ° C to obtain a uniform The solution, sol or suspension is then spray-dried, the inlet air temperature is 150°C, the outlet air temperature is 100°C, the feed rate of the peristaltic pump is 1000mL / h, and finally the obtained product material is kept at 800°C for 8 hours in an air atmosphere , that is, the walnut-shaped fast ion conductor material Li 1+x al x Ti 2-x (PO 4 ) 3 , the resulting product was pressed into tablets under certain pressure conditions to prepare an all-solid electrolyte, and then assembled with a lithium cobaltate positive electrode and a lithium metal negative electrode to form a button battery. The ...
Embodiment 2
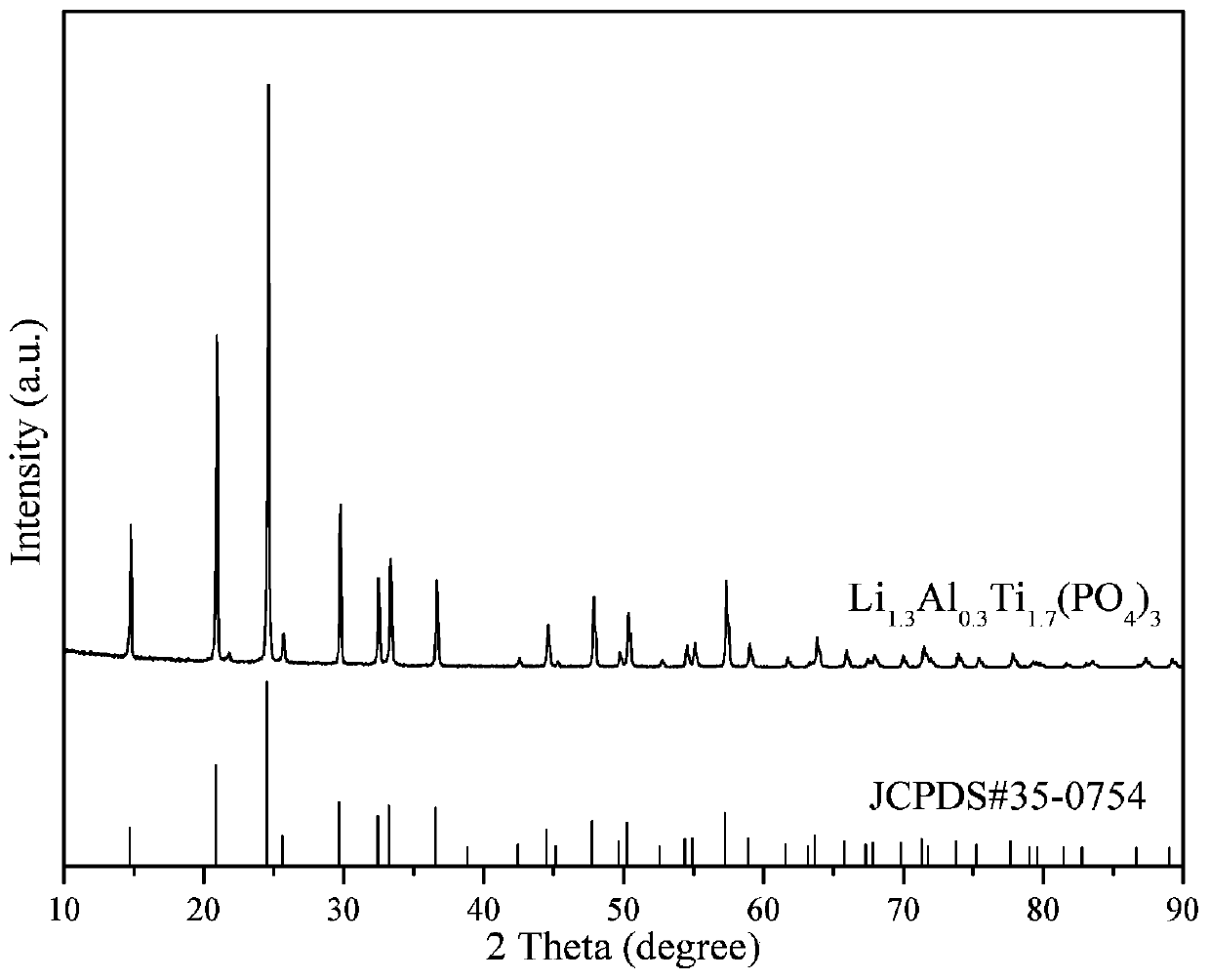
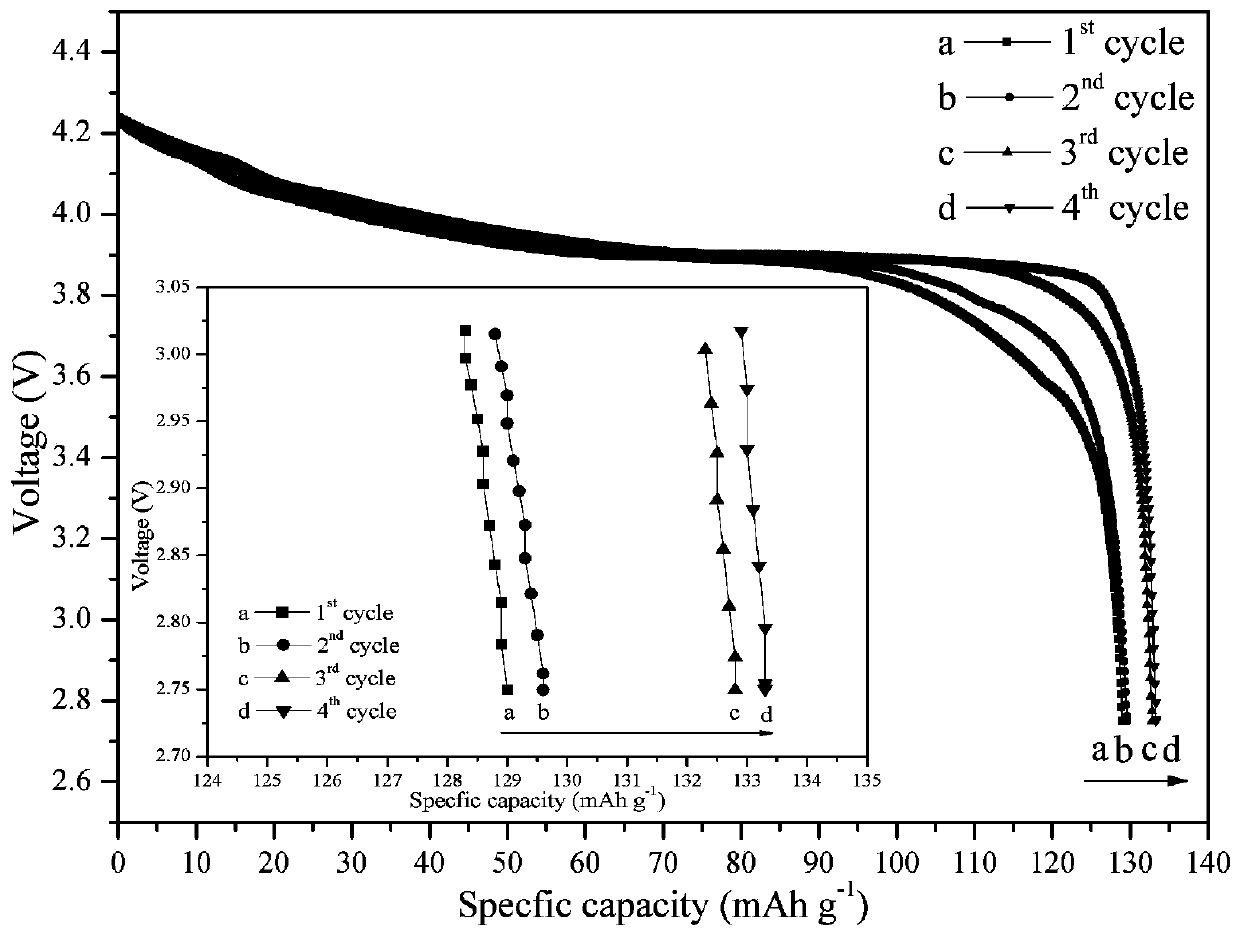
[0034] Using lithium oxalate, aluminum oxalate, n-butyl titanate and ammonium dihydrogen phosphate as raw materials, according to Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 The stoichiometric ratio is mixed in an aqueous solution of ethanol, then acetylacetone is added (according to 2 times the theoretical amount), hydrolyzed for 8 hours under vigorous stirring at 60°C to obtain a uniform solution, sol or suspension, and then spray-dried. The air temperature is 150°C, the outlet air temperature is 100°C, and the feed rate of the peristaltic pump is 1000mL / h. Finally, the obtained product materials are kept at 500, 600, 700, 800, 1000, 1200, and 1300°C for 8 hours in an air atmosphere. The walnut-shaped fast ion conductor material Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 , the resulting product was pressed into tablets under certain pressure conditions to prepare an all-solid electrolyte, and then assembled with a lithium cobaltate positive electrode and a lithium metal negative electrode to...
Embodiment 3
[0039] Using lithium oxalate, aluminum oxalate, n-butyl titanate and ammonium dihydrogen phosphate as raw materials, according to Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 The stoichiometric ratio is mixed in an aqueous solution of ethanol, then acetylacetone is added (according to 2 times the theoretical amount), hydrolyzed for 8 hours under vigorous stirring at 60°C to obtain a uniform solution, sol or suspension, and then spray-dried. The air temperature is 150°C, the outlet air temperature is 100°C, and the feed rate of the peristaltic pump is 1000mL / h. Finally, the obtained product material is kept at 800°C in an air atmosphere for 1, 2, 4, 6, 8, 10, and 11 hours respectively. The walnut-shaped fast ion conductor material Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 , the resulting product was pressed into tablets under certain pressure conditions to prepare an all-solid electrolyte, and then assembled with a lithium cobaltate positive electrode and a lithium metal negative electrode to f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com