Method for producing gamma-aminobutyric acid by whole cell transformation method
A production method and amino acid technology, applied in the biological field, can solve the problems of inability to meet the needs of industrial production, low yield of γ-aminobutyric acid, etc., and achieve the effect of reducing the cost of separation and extraction, single component, and high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The vector construction used in the present invention preferably following method:
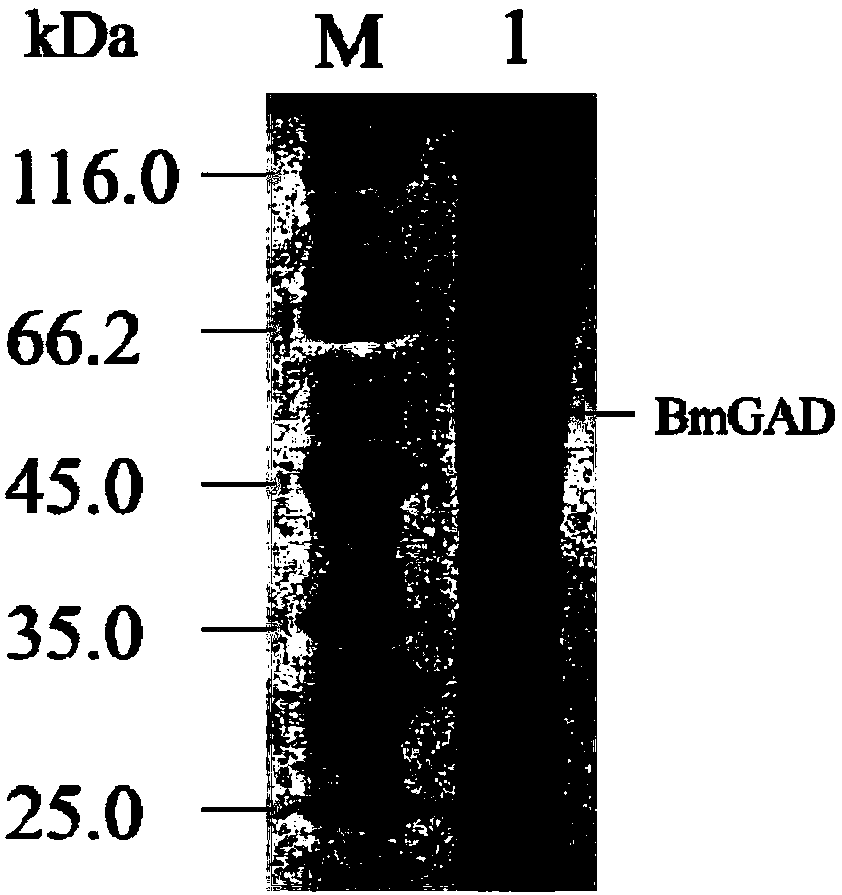
[0059] Guided by primers gad-F (SEQ ID No.3) / gad-R (SEQ ID No.4), preferably using the codon-optimized BmGAD gene (SEQ ID No.2) as a template for PCR amplification reaction, amplified The augmented fragment was digested with HindIII and BamHI and then ligated into the plasmid pXMJ19 digested with HindIII and BamHI to construct the plasmid pXMJ19-BmGAD.
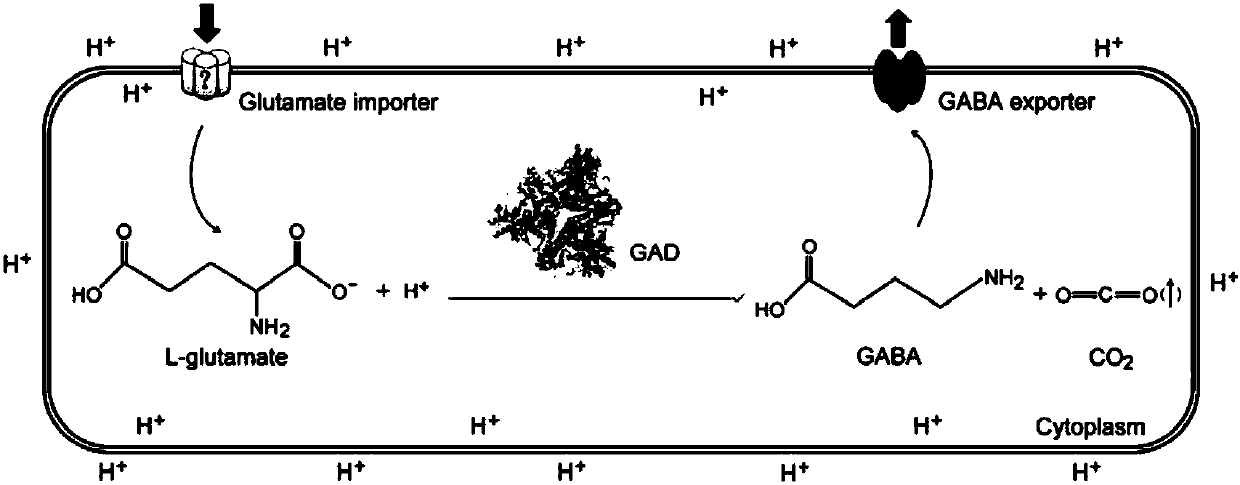
[0060] The vector pXMJ19-BmGAD is transformed into a Corynebacterium glutamicum strain to obtain a Corynebacterium glutamicum strain CG-BmGAD capable of catalytically producing γ-aminobutyric acid in whole cells.
[0061] Preferably, in the present invention, the Corynebacterium glutamicum strain is preferably CG-BmGAD.
[0062] Those skilled in the art should understand that Corynebacterium glutamicum and Bacillus megaterium exhibit different degrees of codon preference when expressing proteins. The codon optimization of the glutamat...
Embodiment 2
[0067] Seed medium: glucose 10-15g / L, corn steep liquor 1-3g / L, ammonium sulfate 10-20g / L, magnesium sulfate 0.5-1g / L, potassium dihydrogen phosphate 0.5-1g / L, sodium citrate 0.5- 2g / L.
[0068] Fermentation medium: glucose 40-60g / L, corn steep liquor 5-8g / L, ammonium sulfate 10-30g / L, magnesium sulfate 0.5-5g / L, potassium dihydrogen phosphate 0.5-5g / L, sodium citrate 0.5- 2g / L.
Embodiment 3
[0070] This example is used to illustrate the high-density fermentation culture of recombinant Corynebacterium glutamicum CG-BmGAD.
[0071] In the present invention, the method for fermenting culture may comprise the following steps:
[0072] (1) Inoculate the recombinant Corynebacterium glutamicum expression strain CG-BmGAD constructed as described above into the seed medium containing chloramphenicol as a selection marker, and cultivate it for 16 hours at 30 degrees Celsius to obtain recombinant glutamic acid Corynebacterium acid CG-BmGAD seed solution;
[0073] (2) Inoculate the recombinant Corynebacterium glutamicum CG-BmGAD seed liquid obtained in step (1) into the fermentation medium containing the chloramphenicol as the screening marker, and cultivate it at 30 degrees Celsius, when the OD 600Add isopropyl-β-D-thiogalactopyranoside (IPTG) when the temperature reaches 5-10, and induce culture at 16-20 degrees Celsius for 3-6 hours.
[0074] Those skilled in the art sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com