Evodiamine derivative, synthetic method and application thereof
A kind of technology of alkaloid derivatives and synthesis method, which is applied in the field of alkaloid derivatives of Evodia rutaecarpa and its synthesis, can solve the problems that the physicochemical properties of anti-tumor drug effects need to be improved, and achieve the goals of inhibiting bacterial growth, high yield, and easy industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
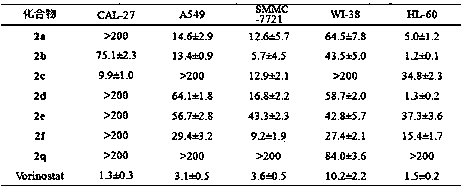
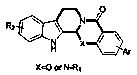
[0038] A method for synthesizing alkaloid derivatives of Evodia rutaecarpa, the specific steps are: at room temperature, 213 mg (1 mmol) of the compound of formula (1), 162 mg (1 mmol) of tryptamine, 1-(3-dimethylaminopropyl)- 573 mg (3 mmol) of 3-ethylcarbodiimide hydrochloride, 101 mg (1 mmol) of triethylamine, 12.2 mg (0.1 mmol) of 4-dimethylaminopyridine, dissolved in 10 mL of dichloromethane, reacted for 5 hours, and extracted The product N-(2-(1H-indol-3-yl)ethyl)-2-aminobenzamide (335mg, yield 90%) was obtained by column chromatography.
[0039]Dissolve 444mg (3mmol) of triethyl orthoformate, 355mg (1mmol) of N-(2-(1H-indol-3-yl)ethyl)-2-aminobenzamide, and 70.5mg (0.5mmol) of boron trifluoride ether in 10mL dimethylformamide. The reaction lasted for 5 hours, and the reaction temperature was 100°C. After the reaction was completed, the product 2a was obtained by separation by extraction column chromatography
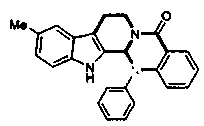
[0040] (R)-14-phenyl-8,13,13b,14-tetrahydroindolo[2',3':3,...
Embodiment 2
[0044] A method for synthesizing alkaloid derivatives of Evodia rutaecarpa, the specific steps are: at room temperature, 213 mg (1 mmol) of the compound of formula (1), 162 mg (1 mmol) of tryptamine, 1-(3-dimethylaminopropyl)- 573 mg (3 mmol) of 3-ethylcarbodiimide hydrochloride, 101 mg (1 mmol) of triethylamine, 12.2 mg (0.1 mmol) of 4-dimethylaminopyridine, dissolved in 10 mL of dichloromethane, reacted for 5 hours, and extracted The product N-(2-(5-methyl-1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide (348mg, yield 90%) was obtained by column chromatography.
[0045] Triethyl orthoformate 444mg (3mmol),
[0046] N-(2-(5-methyl-1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide369mg (1mmol), boron trifluoride ether 70.5mg (0.5mmol) dissolved in 10mL dimethylformamide . The reaction lasted for 5 hours, and the reaction temperature was 100°C. After the reaction was completed, the product 2 b was obtained by separation by extraction column chromatography
[0047] (R)-10-methyl-14-...
Embodiment 3
[0051] A method for synthesizing alkaloid derivatives of Evodia rutaecarpa, the specific steps are: at room temperature, 213 mg (1 mmol) of the compound of formula (1), 162 mg (1 mmol) of tryptamine, 1-(3-dimethylaminopropyl)- 573 mg (3 mmol) of 3-ethylcarbodiimide hydrochloride, 101 mg (1 mmol) of triethylamine, 12.2 mg (0.1 mmol) of 4-dimethylaminopyridine, dissolved in 10 mL of dichloromethane, reacted for 5 hours, and extracted The product N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide (362mg, yield 90%) was obtained by column chromatography.
[0052] Triethyl orthoformate 444mg (3mmol),
[0053] N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide385mg (1mmol), boron trifluoride ether 70.5mg (0.5mmol) dissolved in 10mL dimethylformamide . The reaction lasted for 5 hours, and the reaction temperature was 100°C. After the reaction was completed, the product 2c was separated by extraction column chromatography
[0054] (R)-10-methoxy-14-phenyl-8,13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com